REF: LIB-1, LIB-3 REF: LIB-1, LIB-3
LiNA Librata™ LiNA Librata™
Original IFU
INDICATION FOR USE:
The LiNA
Librata
is a thermal balloon ablation
device intended to ablate the endometrial lining
of the uterus in pre-menopausal women with
menorrhagia (excessive uterine bleeding) due to
benign causes for whom childbearing is complete.
CONTRAINDICATIONS:
The device is contraindicated for use in:
• A patient who is pregnant or who wants to
become pregnant in the future. Pregnancies
following ablation can be dangerous for both
mother and fetus.
• A patient with any anatomic condition (e.g.,
history of previous classical cesarean sections
or transmural myomectomy) or pathologic
condition (e.g., chronic immunosuppressive
therapy) that could lead to weakening of the
myometrium.
• A patient with known or suspected endometrial
carcinoma (uterine cancer) or premalignant
change of the endometrium, such as unresolved
adenomatous hyperplasia.
• A patient with an intrauterine device (IUD)
currently in place.
• A patient with active genital or urinary tract
infection at the time of procedure (e.g., cervicitis,
vaginitis, endometritis, salpingitis, or cystitis) or
with active pelvic inflammatory disease (PID).
• Repeated ablation should not be performed
using global ablation but only by hysteroscopic
ablation by an experienced surgeon.
WARNINGS:
Failure to follow all instructions or to heed any
warnings or precautions could result in serious
patient injury.
• The LiNA Librata is provided STERILE. Carefully
inspect the packaging for any damage prior to
use. Do NOT attempt to use the device if sterile
barrier is damaged. Do NOT use past expiration
date. Do NOT use if the device is exposed to non-
sterile surfaces before procedure.
• For single use only. Do NOT reuse, reprocess or
re-sterilize the LiNA Librata. Any reprocessing
may impede the functions of this device.
Reusing single use devices may also increase the
risk of cross contamination. Attempts to clean
the device results in risk of device malfunction
and/or erroneous pathology specimen collection
due to residual tissue in the LiNA Librata.
• No modification of this equipment is allowed.
• The ambient temperature must not be higher
than 30°C during the use of this equipment.
• The device is intended for use only in women
who do not desire to bear children because
the likelihood of pregnancy is significantly
decreased following this procedure. There have
been reports of women becoming pregnant
following this procedure. Pregnancies after
ablation can be dangerous for both mother and
fetus.
• Endometrial ablation using the LiNA Librata is
not a sterilization procedure. The patient should
be advised of appropriate birth control methods.
• Endometrial ablation procedures using the
LiNA Librata should be performed only by
medical professionals who have experience in
performing procedures within the uterine cavity,
such as IUD insertion or dilation and curettage
(D&C), and who have adequate training and
familiarity with the LiNA Librata.
• Do NOT perform same-day LiNA Librata
procedures and hysteroscopic tubal occlusion/
sterilization. Ablation may cause intrauterine
synechiae, which can compromise (i.e.,
prevent) the 3-month confirmation test (HSG)
for the tubal occlusion device. Women who
have inadequate 3-month confirmation tests
cannot rely on the tubal occlusion device for
contraception.
• Patients who undergo endometrial ablation
procedures who have previously undergone
tubal ligation are at increased risk of developing
post-ablation-tubal sterilization syndrome,
which can require hysterectomy. This can occur
as late as 10 years post-procedure.
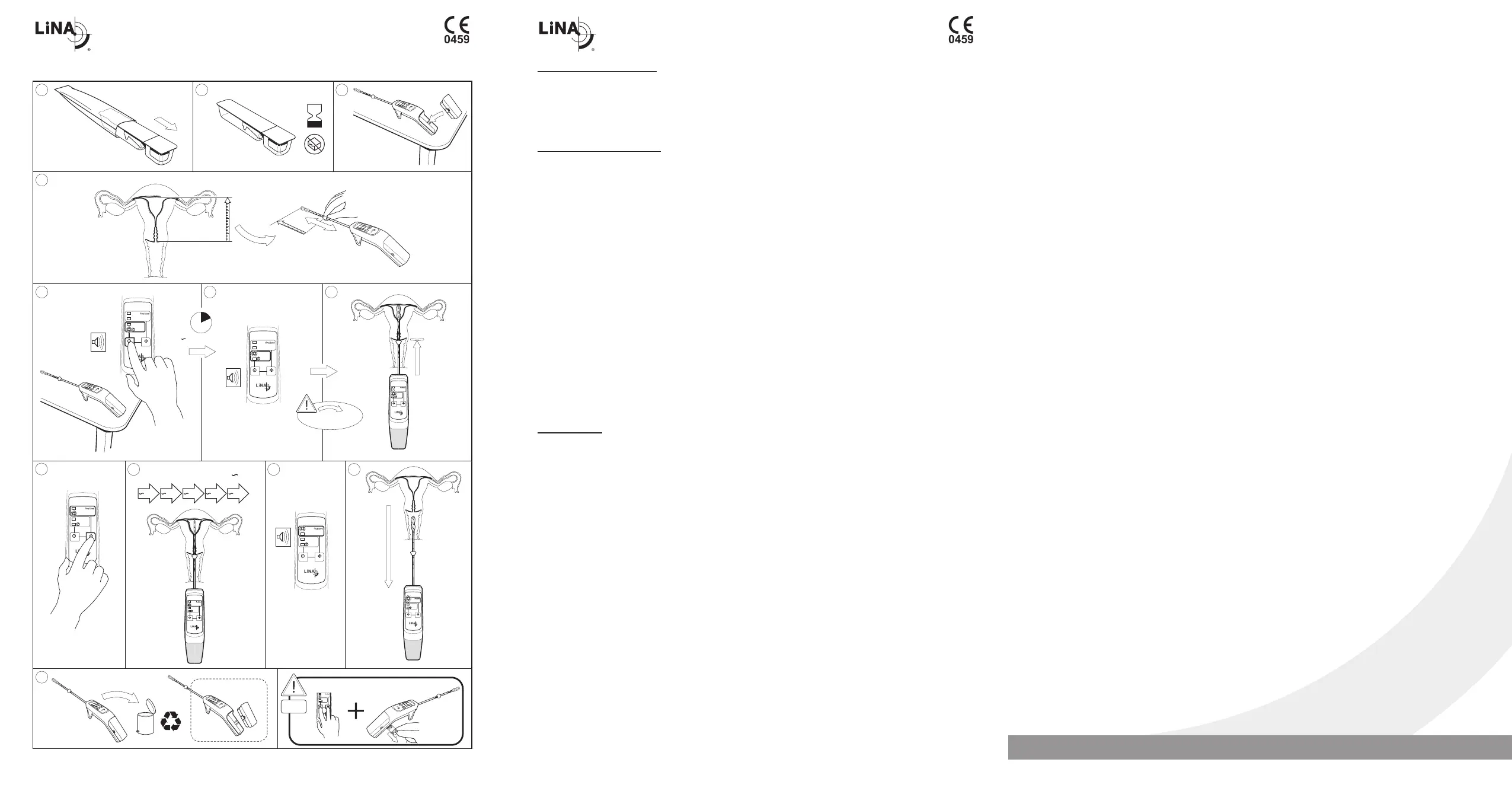
• Uterine perforation can occur during any
procedure in which the uterus is instrumented.
Use caution not to perforate the uterine wall
when sounding the uterus, dilating the cervix,
curretaging the uterine lining or inserting the
catheter. If uterine perforation is suspected,
THE PROCEDURE SHOULD BE TERMINATED
IMMEDIATELY and hysteroscopy should be
performed to assess the uterine cavity.
• If a perforation is present, and the procedure is
not terminated, thermal injury to adjacent tissue
may occur.
• Endometrial ablation procedures do not
eliminate the potential for endometrial
hyperplasia or adenocarcinoma of the
endometrium and may mask the physician’s
ability to detect or make a diagnosis of such
pathology.
• Once treatment is initiated in the uterus, patients
are to be treated using 1 LiNA Librata device.
LiNA Librata delivers an automated, 5 treatment
cycle therapy. If the treatment is interrupted
during any of the 5 cycles, DO NOT treat with
any additional devices because of the potential
for injury. Do not treat patients for more than
one therapy cycle in a given treatment session
because of the potential for transmural injury to
the uterus or injury to adjacent viscera.
• Portable RF communications equipment
(including peripherals such as antenna cables
and external antennas) should be used no
closer than 30 cm (12 inches) to any part of
LiNA Librata. Otherwise, degradation of the
performance of this equipment could result.
• The balloon must not come into contact with
sharp edges
• The distal end of the housing can reach
temperatures of up to 48°C and should not be
held.
• The balloon is very hot during and right after
treatment.
• Do not remove the balloon catheter during the
treatment process.
5
6
7
8
9 11
12
treating
ready
heating
on
abort
treat
treating
ready
heating
on
abort
treat
treating
ready
heating
on
abort
treat
treating
ready
heating
on
abort
treat
treating
ready
heating
on
abort
treat
(x) cm
(x) cm
treating
ready
heating
on
abort
treat
treating
ready
heating
on
abort
treat
10 min.
max. 30 min.
2 min.
treating
ready
heating
on
abort
treat
25s 25s 25s 25s 25s
abort
A-D E-F
1
2
3
4
10
Innovation in Gynecology
 Loading...
Loading...