12
PM-1655-E-01-01/2018
Packaging specifications
List of Harmonized EN Standards
The device is classified as a medical device, class IIa.
Applicable Medical Device Directive (93/42/EEC) standards:
EN 13544-1:2009
EN 15223-1:2016
INI EN 1041:2013
UNI EN ISO10993-1:2010
EN 10993-5:2009
EN 10993-10:2010
EN 60 601-1:2006+A1 :2013 (Ed3.1)
EN 60 601-1-2:2015 4
th
edition
EN 60 601-1-11:2015
EN 60 601-1-6:2010
EN 62366:2008
EN 14971:2012
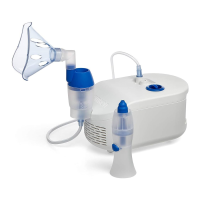
Operation and care
Cleaning of the device
Clean the parts after each use to remove residual medication or mucus. This will
prevent inefficient nebulization or aspiration and reduce the risk of infection.
Package Amount Approx. weight
Approx. dimensions
W x D x H mm
Main Unit 1 1.1 kg 145 x 222 x 124 mm
Package 1 1.45 kg 152 x 233 x 181 mm
Master Carton 6 9.75 kg 330 x 722 x 210 mm
EAN code 401567211121 9
 Loading...
Loading...