63
3
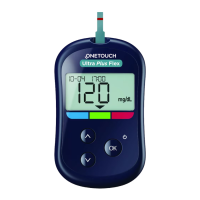
Taking a test
• When you first open a new vial of control solution,
record the discard date on the vial label. Refer to the
control solution insert or vial label for instructions on
determining the discard date.
• Tightly close the cap on the control solution vial
immediately after use to avoid contamination or
damage.
See page36 for more information on testing.
CAUTION:
• Do Not swallow or ingest control solution.
• Do Not apply control solution to the skin, eyes, ears or
nose as it may cause irritation.
• Do Not use control solution after the expiry date
(printed on the vial label) or the discard date,
whichever comes first, or your results may be
inaccurate.
 Loading...
Loading...