18
BLUETOOTH MODULES REGULATED INFORMATION
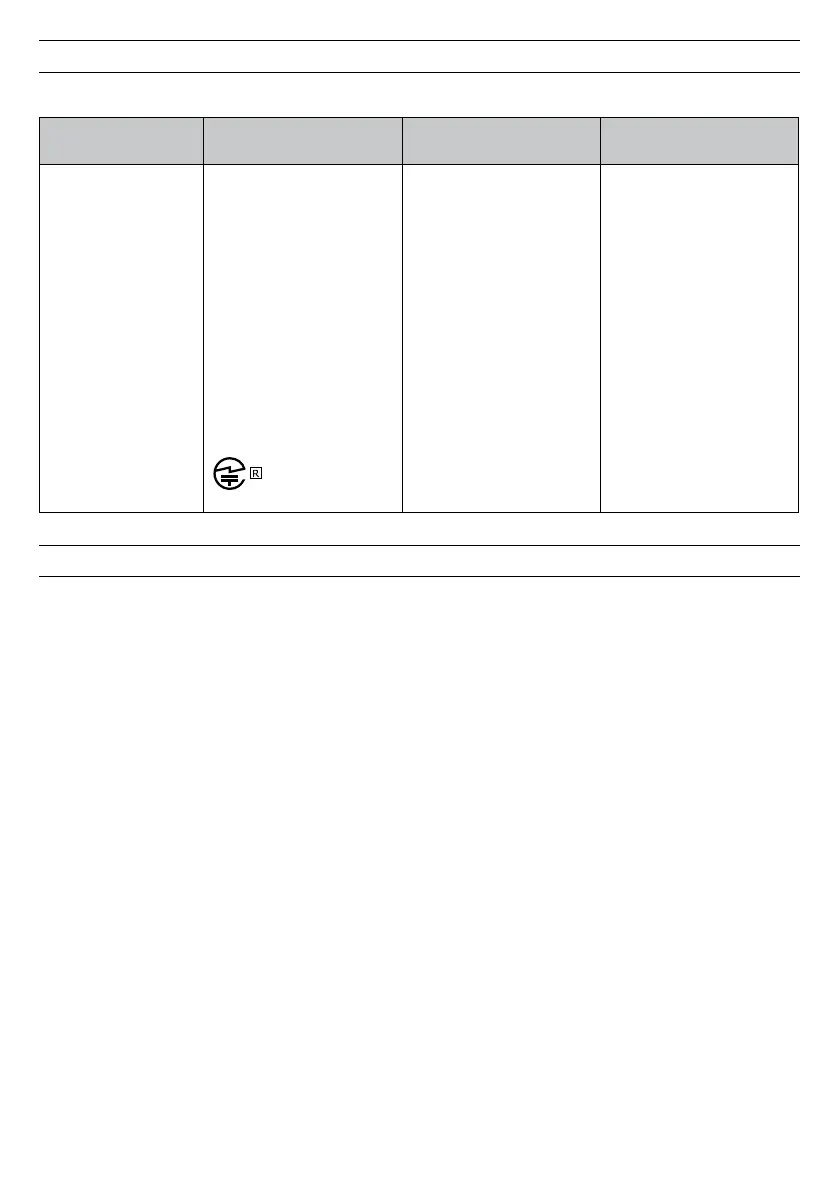
This device contains the following radio frequency transmitters:
Model Re
Type and Frequency
Characteristics
Effective Radiated Power
Bluetooth Low Energy
Dual Mode Module
Model BR-LE.-DA
FCC
Contains FCC ID:
XDULE-D
Canada
Contains IC: A-LED
Japan
Contains transmitter with
certificate number
(Dual Mode)
Version V. +ED (GFSK +
π/ DQPSK + DPSK)
- MHz
Version V. (GFSK)
- MHz
Adjustable Power (- dBm
to . dBm) short to
long range
ELECTROMAGNETIC COMPATIBILITY
WARNING: Use of this equipment adjacent to or stacked with other equipment should be avoided because it could
result in improper operation. If such use is necessary, this equipment and the other equipment should be
observed to verify that they are operating normally.
WARNING: Use of accessories, transducers and cables other than those specified or provided by the manufacturer
of this equipment could result in increased electromagnetic emissions or decreased electromagnetic immunity
and result in improper operation
In order to regulate the requirements for Electromagnetic Compatibility (EMC) with the aim to prevent unsafe
product situations, the BS EN --:/ IEC --: standard has been implemented. This standard
defines levels of electromagnetic emissions for medical devices.
The Össur Myoelectric Prosthetic Devices are manufactured by Össur hf. conforms to this BS EN -- :/
IEC --: standard for both immunity and emissions.
The i-Limb hand is suitable for use in any environment except where immersion in water or any other fluid is
possible, or where exposure to highly electrical and/or magnetic fields can occur (e. g. electrical transformers,
high-power radio/TV transmitters, RF surgical equipment, CT and MRI scanners).
Refer to further guidance below regarding the EMC environment in which the device should be used:
205-160268

 Loading...
Loading...











