- 32 -
4-1-4 HIGH-LEVEL DISINFECTION
Before any attempt is made to disinfect the endoscope, the complete cleaning procedure described elsewhere in
this manual must have been completed. Prior to high-level disinfection, the end user should confirm the minimum
effective concentration (MEC) of reused disinfectant as per the manufacturer’s instructions. Before complete im-
mersion in any disinfecting solution the endoscope should have been “Leak Tested” as described elsewhere in this
manual.
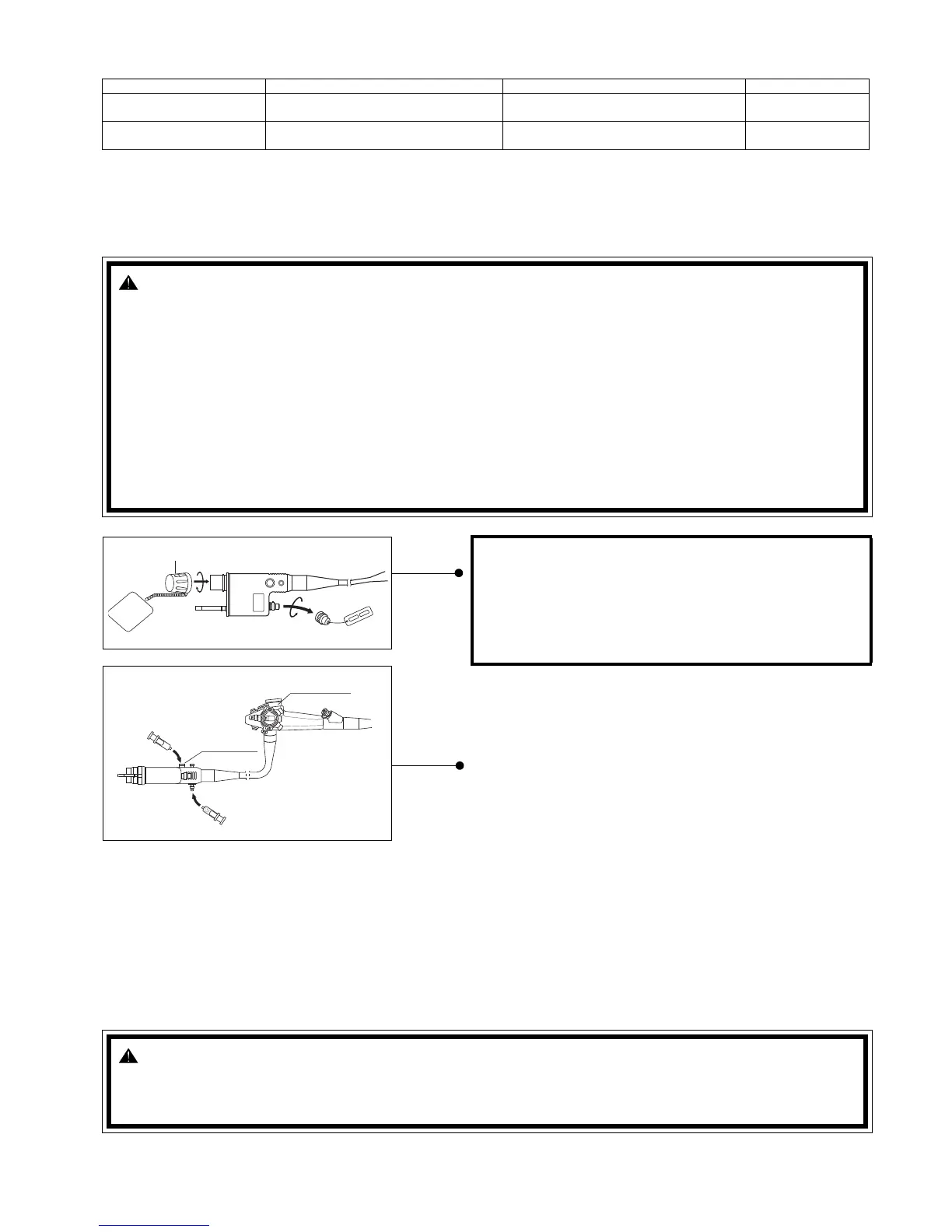
1) The air/water/instrument channel disinfecting adapters
consisting of two separate components OF-B115 and
OF-G17 should already be installed on the endoscope
from the previous cleaning procedure.
a) Model number OF-G17 incorporates a standard ANSI
luer lock connector to which a syringe or other device
should be attached. Fresh (or reused actively effective)
disinfecting solution should be flushed through this
connector and will simultaneously flow through both
the air and water channels of the scope. (Refer to the internal schematics)
b) After the entire scope is completely immersed, and the air and water channels have been filled with disinfect-
ing solution, Model Number OF-G17 should be removed.
c) Adhere to proper exposure times for the disinfectant.
d) Confirm that a rubber inlet seal is already attached to each channel inlet during the next step.
e) The suction nipple located on the PVE connector incorporates a standard luer slip fitting to which a syringe or
other device can be attached. Fresh (or reused actively effective) disinfecting solution should be flushed
through or drawn into the entire suction system.
Channel Adapters/Components to be attached Disinfecting solution Rinse water
Air/Water Channel OF-B115
OF-G17
50 mL or more 70 mL or more
Biopsy/Suction Channel OF-B115
Rubber Inlet Seal
100 mL or more 150 mL or more
WARNING:
It is imperative that flexible endoscopes and other semi-critical devices be reprocessed using at least
high-level disinfection with a legally marketed sterilant/disinfectant. Only legally marketed endoscope
automated reprocessing devices/systems whose device specific claims have been validated by the
AER/WD manufacturer and/or reprocessing agents which have been tested and found to be compatible
by PENTAX should be used with PENTAX products.
Generally speaking, “2%” and “3.2%” alkaline glutaraldehyde solutions which have been FDA cleared
with High-Level Disinfection and/or Sterilization claims are recommended. It should be noted that the
actual percentage of active ingredient (glutaraldehyde) in these solutions, as per their product label,
may vary from the generic and traditional terms “2% glutaraldehyde” and/or “3.2% glutaraldehyde”.
For specific brands of compatible disinfectants/sterilants, please contact your local PENTAX service
facility or sales representative. Please also refer to the inside front cover of this manual regarding infec-
tion control.
ETO Cap
PVE Soaking Cap
(OF-G17)
(OF-B115)
CAUTION:
BEFORE IMMERSING, confirm that
a) The ‘Red’ ETO gas sterilization venting cap is
taken OFF.
b) The PVE soaking cap is securely ON the elec-
trical contacts.
WARNING:
Avoid introduction of air during the flushing process. Confirm that no air bubbles exit from the channel
openings at the scope distal tip. The presence of the air bubbles could prevent contact of the disinfect-
ant with channel surfaces.

 Loading...
Loading...