Page 11/14
6. Technical specifications
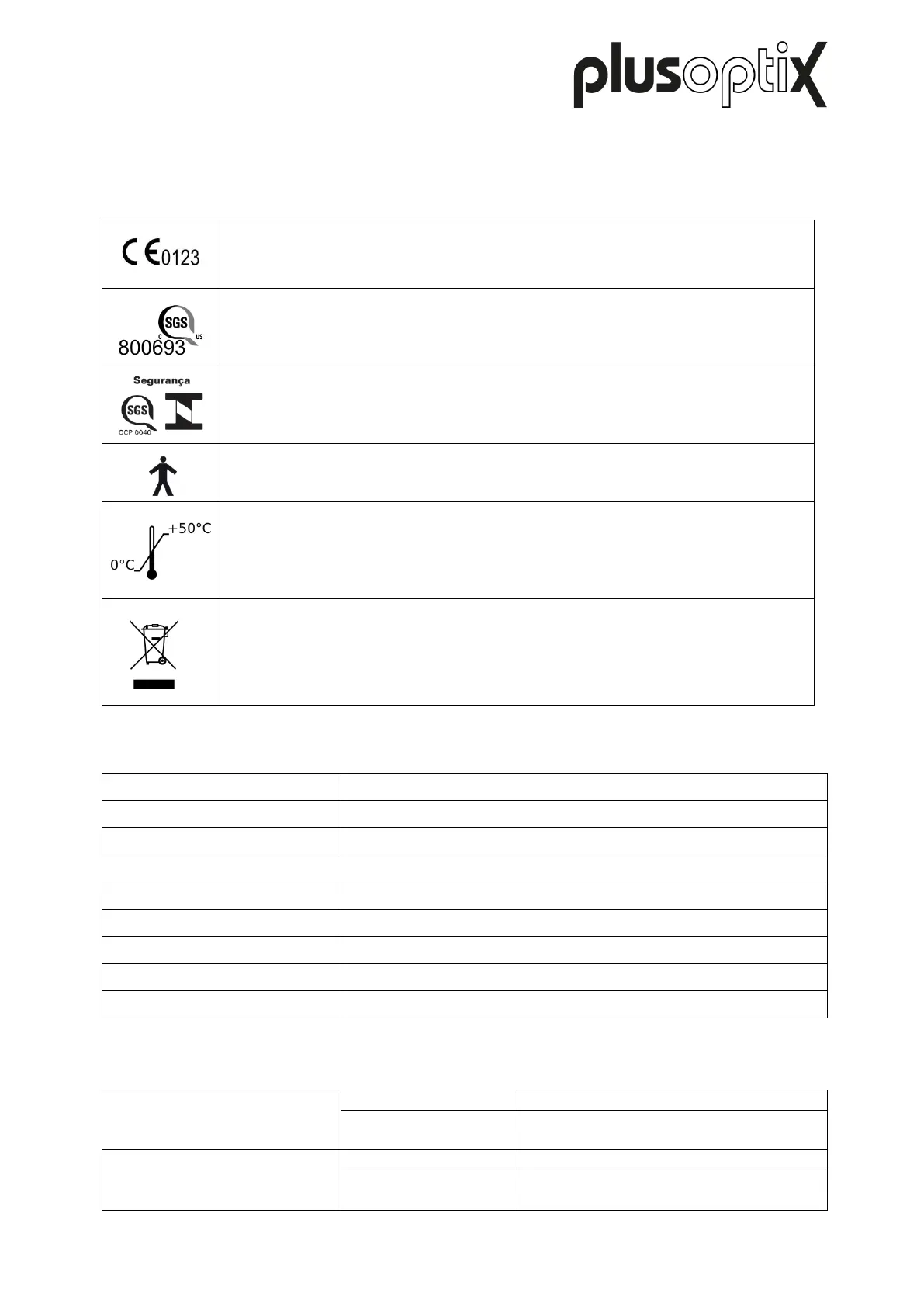
Requirements & directives
The device meets the requirements of Medical Devices Directive
2007/47/EC.
The device has been tested and certified for compliance with applicable US
American and Canadian standards by the Nationally Recognized Test
Laboratory (NRTL).
The device has been certified according to Brazilian requirements and may
therefore bear the INMETRO certification mark.
The device meets the requirements for a Type B application part as per IEC
60601-1.
The device can be stored and transported at temperatures between 0°C to
+50°C (i.e 32°F to 122°F). Operation requires a temperature between +10°C
and +40°C (i.e. 50°F to 104°F) at 20% to 80% relative humidity (non-
condensing).
Disposal
Do not dispose of the device as domestic waste. Please send the device to
Plusoptix for environmentally sound disposal. We will reimburse you for the
cost of the return.
Measurement values
Measuring range and tolerance
-7 to +5 dpt in 0.25 dpt steps ± 0.25 dpt
-7 to +5 dpt in 0.25 dpt steps ± 0.25 dpt
1 to 180° in 1° steps ± 15°
3 to 8 mm in 0.1 mm steps ± 5%
25 to 85 mm in 0.1 mm steps ± 5%
0 to 25° in 0.1° steps ± 2.62°
-71 to +71° in 1° steps ± 0.75°
-30 to +30° for 5 mm pupil size; in 0.1° steps ± 1,85°
Power supply
Power supply
(medical power adapter
GSM36B12-P1J)
100–240 V AC, 50/60 Hz, 0.9–0.45 A
Nickel metal hydride NiMH / AA HR6
1,900 to 2,100 mAh / 6 pieces

 Loading...
Loading...