20 21
Name of product Revitive Essential
Model RLV
Frequency (+/-10%)
Foot: 20 to 44Hz
Body: 37 to 51Hz
Output current Max 8.5mA
Weight (+/-0.5kg) 1.13kg
Dimensions Ø 355 mm x 77(D) mm
Power consumption 5W
AC adaptor
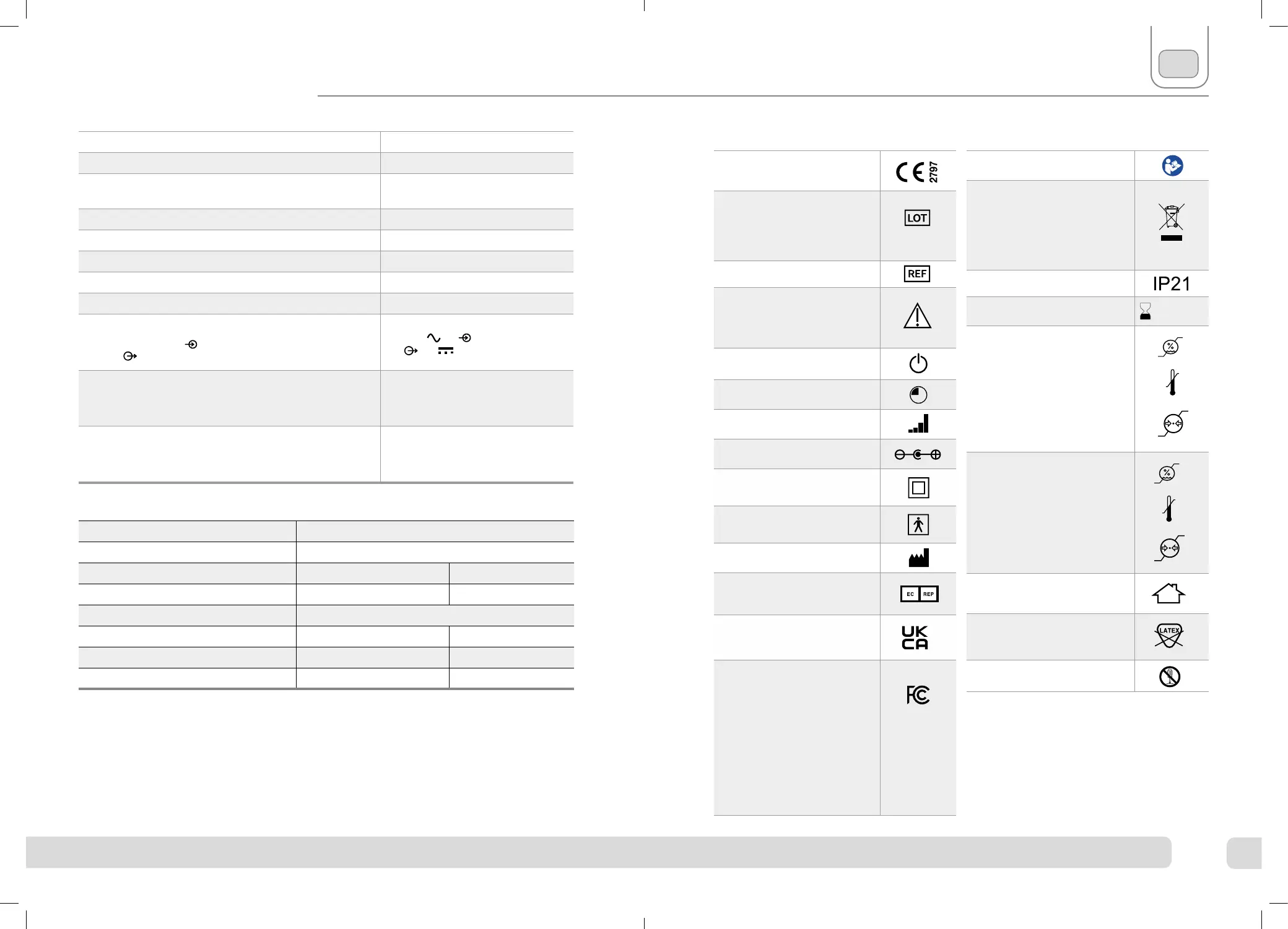
CE Approved
Power source
Input (adaptor used)
Output
100-240V AC ( ), 50/60Hz, 0.18A
5V ( ) DC ,1.0A
Service Life of Device
Period of time specified by the manufacturer during which the device
is expected to remain safe for use.
4 Years
Applied parts
Part of Revitive that in normal use come into physical contact with
user to perform therapy
Foot-pads - 135.5cm
2
Electrode Body Pads - 5cm x 5cm = 25cm
2
Technical specifications
The values of PULSE DURATIONS, PULSE repetition frequencies and amplitudes, including any d.c. component, shall not
deviate by more than ± 20 % when measured with a load resistance within the range specified.
If confirmation is required that the Revitive works within its Essential Performance aer a certain period of time, contact
the manufacturer
Output Specifications for Electrical Muscle Stimulation (EMS):
Waveform Biphasic
Shape Square symmetrical with polarity reversal
Maximum Output Voltage (+/-15%) @500Ω Foot: 21.5Vp Body: 21.5Vp
Pulse Duration (+-10%) Foot: 450 or 970µs (Mode 7) Body: 450µs
Net Charge @ 500Ω [0.001]mC
Maximum Power Density @ 500Ω Foot: 0.19 mW/cm² Body: 1.50 mW/cm²
ON Time (+-10%) Foot: 1.9 - 8.3s Body: 1.9 - 6.5s
OFF Time (+-10%) Foot: 1.00 - 1.50s Body: 1.00s
EN
Complies with European Medical
Devices Directive (93/42/EEC)
Device serial number including
year (YYYY) and month (MM) of
manufacture can be found on the
box and back of unit
Item number
Contraindications, Warnings and
Cautions
Make sure you understand these before using
Revitive
Power
Time Remaining
Intensity Level
Center Positive Polarity
Class II medical electrical
equipment double insulated
Type BF medical electrical
equipment
Legal manufacturer of the device
EU/EC European Authorized
Representative
UK Conformity Assessed
Product conforms to all applicable U.K. legislative
requirements.
FCC mark
Certification mark employed on electronic
products manufactured or sold in the United
States which certifies that the electromagnetic
interference from Revitive is under limits
approved by the Federal Communications
Commission. Actegy Ltd complies with all
applicable FCC rules.
#YYYYMMXXXXX
Symbols
Consult instructions for use
The Waste Electrical and Electronic
Equipment Directive (WEEE
Directive).
At the end of the product lifecycle, do not throw
this product into normal household garbage, but
take it to a collection point for the recycling of
electronic equipment
Ingress Protection Rating
Use-by date YYYY MM DD
Humidity, temperature and air
pressure limit for storage and
transport
Humidity, temperature and air
pressure limit for operating
conditions
Aer any exposure to hot or cold temperatures
outside the specified operating range of
10 - 40°C allow the product to re-adjust to the
recommended operating temperatures to ensure
continued product performance.
Indoor Use Only
Medical device does not contain
natural rubber latex
Do not disassemble
-20°C
20%
70°C
90%
500 hPa
1060 hPa
10°C
30%
40°C
75%
700 hPa
1060 hPa
8034_IFU01_18976655.indd 20-21
8034_IFU01_18976655.indd 20-21
08/02/2023 09:22
 Loading...
Loading...