Document Name
User Manual
Document No. TG-TPP-001
Model
Theragun PRO PLUS
Rev
01
Page 18 of 24
level
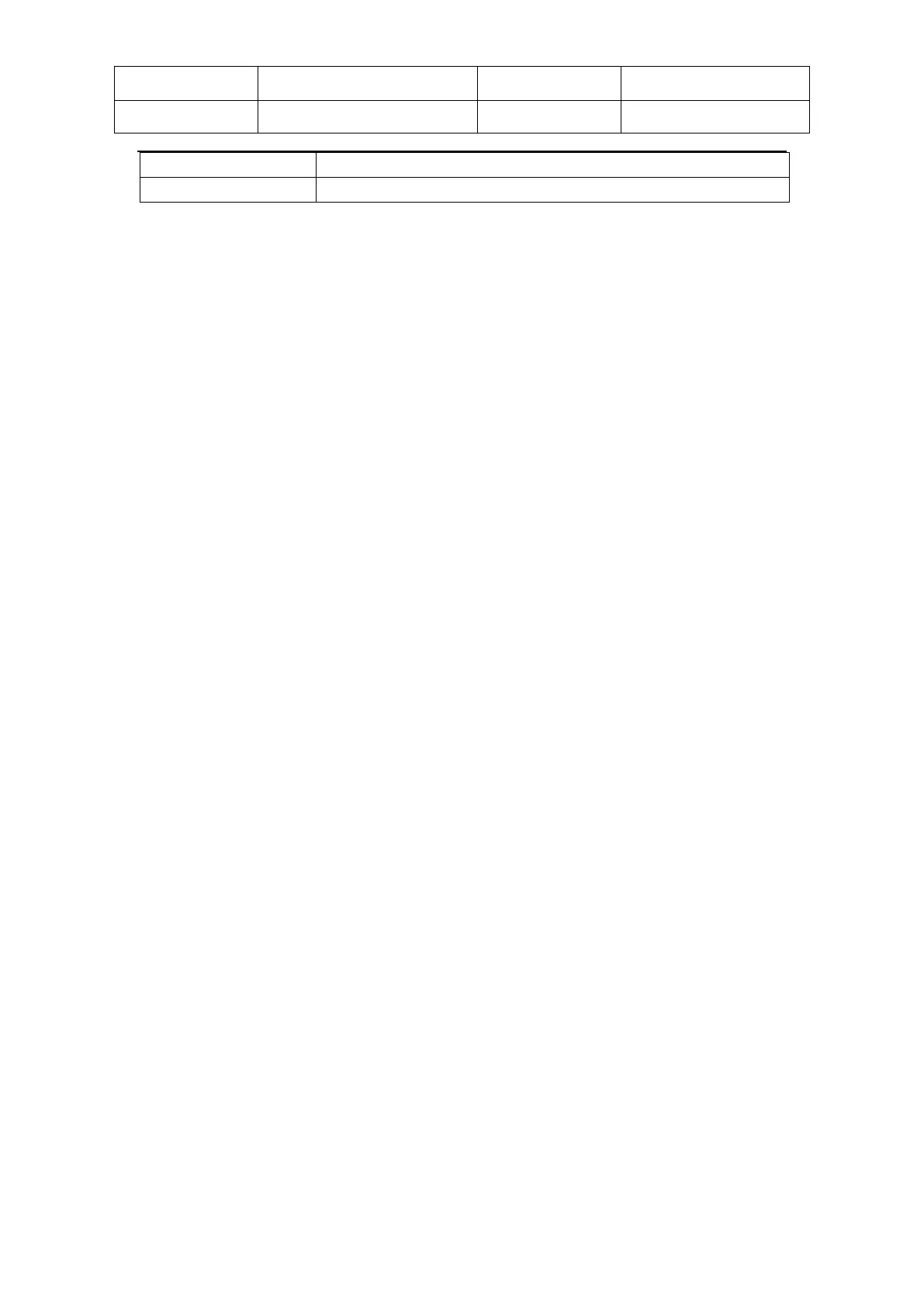
LED module Wavelength: IR=840~860nm; RED=650~670nm; Level=ON/OFF.
8. Safety, EMC
This device is Class II equipment with type BF applied. It complies with
Medical Electrical Safety Standards (IEC 60601-1).
This device also complies with Medical EMC Standard (IEC 60601-1-2).
The has been tested and found to comply with the electromagnetic compatibility
(EMC) limits for medical devices to IEC 60601-1-2: 2007. These limits are
designed to provide reasonable protection against harmful interference in a
typical medical installation.
CAUTION:
Do not apply the device near any devices with Electromagnetic Interference
(EMI), such as cell phones, Magnetic Resonance Imaging (MRI), computerized
axial tomography (CT), diathermy, Radio Frequency Identification (RFID), etc.
or MR environment. EMI, RF devices or MR environment may affect the normal
function of the device or would cause user injury.
The patient is an intended operator. The ME EQUIPMENT shall not be
serviced or maintained while in use with a patient.
Manufacturer’s declaration – electromagnetic immunity
1* WARNING: Use of this equipment adjacent to or stacked with other
equipment should be avoided because it could result in improper operation. If
such use is necessary, this equipment and the other equipment should be
observed to verify that they are operating normally.”
2* WARNING: Use of accessories, transducers and cables other than those
specified or provided by the manufacturer of this equipment could result in
increased electromagnetic emissions or decreased electromagnetic immunity
of this equipment and result in improper operation.”
3* WARNING: Portable RF communications equipment (including peripherals
such as antenna cables and external antennas) should be used no closer
than 30 cm (12 inches) to any part of the ME EQUIPMENT, including cables
specified by the manufacturer. Otherwise, degradation of the performance of
this equipment could result.”
 Loading...
Loading...