Instruction for use
16
En
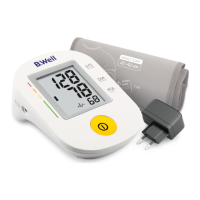
PRO-36
10. APPLIED STANDARDS
The digital automatic blood pressure monitor corresponds to the below standards:
IEC 60601-1:2005/EN 60601-1:2006/AC:2010 (Medical electrical equipment – Part 1: General requirements for basic safety and essential
performance),
IEC60601-1-2:2007/EN 60601-1-2:2007 /AC:2010 (Medical electrical equipment – Part 1-2: General requirements for basic safety and
essential performance – Collateral standard: Electromagnetic compatibility – Requirements and tests),
IEC 80601-2-30 : 2009+Cor.2010 (Medical electrical equipment – Part 2-30: Particular requirements for the basic safety and essential
performance of automated non-invasive sphygmomanometers),
EN 1060-1: 1995 + A1: 2002 + A2: 2009 (Non-invasive sphygmomanometers – Part 1: General requirements),
EN 1060-3: 1997 + A1: 2005 + A2: 2009 (Non-invasive sphygmomanometers – Part 3: Supplementary requirements for electro-mechani-
cal blood pressure measuring systems).
11. SYMBOL INFORMATION
THE OPERATION GUIDE MUST BE READ
(The sign background colour: blue.The sign graphical symbol: white)
WARNING
TYPE BF APPLIED PARTS (The cuff is type BF applied part)
ENVIRONMENT PROTECTION – Waste electrical products should not be disposed of with household waste.
Please recycle where facilities exist. Check with your local Authority or retailer for recycling advice.
MANUFACTURER’S NAME
ARTICLE NUMBER
SERIAL NUMBER
CE mark (0044) COMPILES WITH MDD93/42/EEC REQUIREMENTS
0044
 Loading...
Loading...