(1)
Instructions for Use:
Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
A. Description:
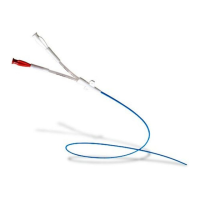
The Bard
®
Max•Core
®
Biopsy Instrument is a single use core biopsy device. It is available in several needle gauge sizes and lengths. The side and
rear actuator buttons are color coded according to the various gauge sizes, e.g., Yellow=20 gauge, Pink=18 gauge, Purple=16 gauge and Green=14
gauge.
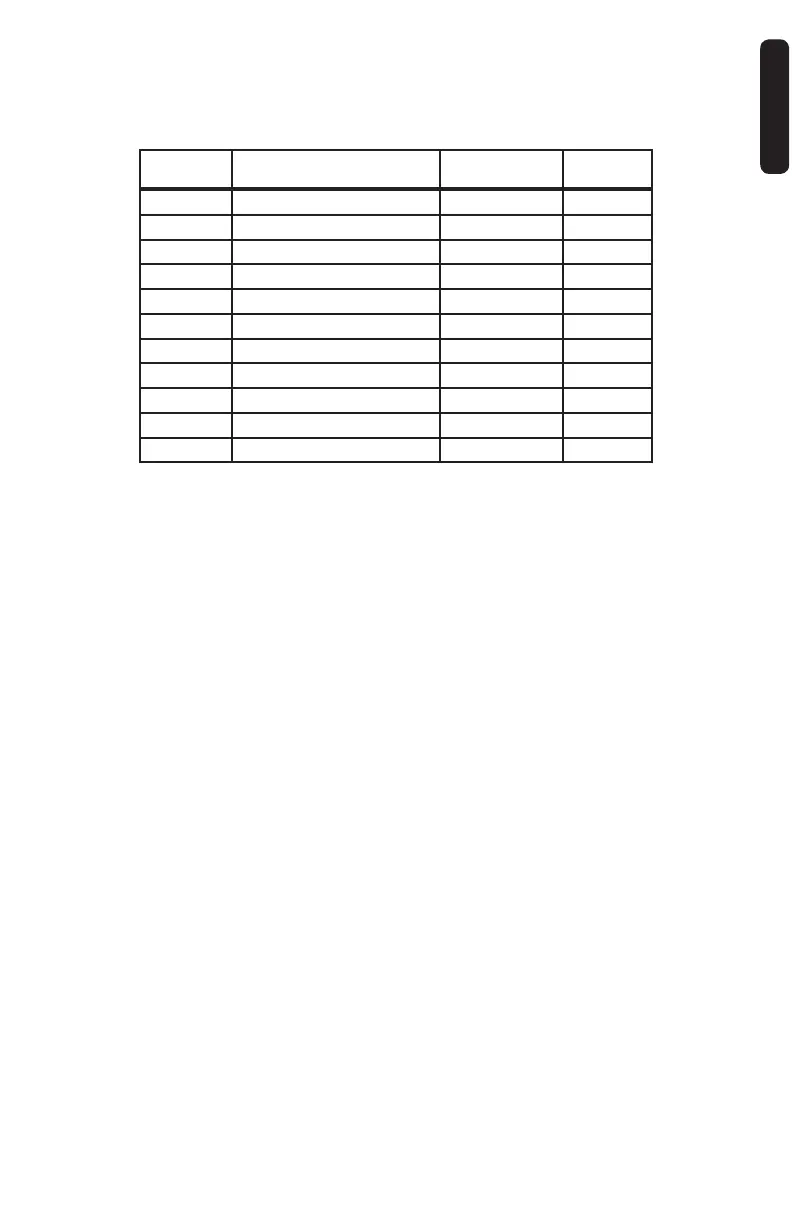
Catalog
Number
Gauge Size and Needle Length
Length of Sample
Notch
Penetration
Depth
MC1410 14g (2.1mm) x 10cm (100mm) 1.9cm (19mm) 22mm
MC1416 14g (2.1mm) x 16cm (160mm) 1.9cm (19mm) 22mm
MC1610 16g (1.7mm) x 10cm (100mm) 1.9cm (19mm) 22mm
MC1616 16g (1.7mm) x 16cm (160mm) 1.9cm (19mm) 22mm
MC1810 18g (1.2mm) x 10cm (100mm) 1.8cm (18mm) 22mm
MC1816 18g (1.2mm) x 16cm (160mm) 1.8cm (18mm) 22mm
MC1820 18g (1.2mm) x 20cm (200mm) 1.8cm (18mm) 22mm
MC1825 18g (1.2mm) x 25cm (250mm) 1.8cm (18mm) 22mm
MC2010 20g (0.9mm) x 10cm (100mm) 1.8cm (18mm) 22mm
MC2016 20g (0.9mm) x 16cm (160mm) 1.8cm (18mm) 22mm
MC2020 20g (0.9mm) x 20cm (200mm) 1.8cm (18mm) 22mm
B. How Supplied:
The product is supplied sterile and nonpyrogenic unless the package has been opened or damaged. Sterilized by using Ethylene Oxide. For sin-
gle use only. Do Not Reuse. Do Not Resterilize.
C. Indications for Use:
The core needle biopsy device is intended for use in obtaining biopsies from soft tissues such as liver, kidney, prostate, spleen, lymph nodes and
various soft tissue tumors.
D. Contraindications:
Not intended for use in bone.
E. Warnings:
1. Good medical judgment should be exercised in considering biopsy on patients who are receiving anticoagulant therapy or who have
bleeding disorders.
2. Post-biopsy patient care may vary with the biospy technique utilized and the individual patient's physiological condition.
Observation of vital signs and other precautions should be taken to avoid and/or treat potential complications that may be associated
with biopsy procedures.
3. The collection of multiple needle cores may help to ensure the detection of any cancer tissue. A "negative" biopsy in the presence of
suspicious radiographic findings does not preclude the presence of carcinoma.
Note: If collecting multiple samples, inspect the needle for damaged point, bent shaft or other imperfections after each sample is col-
lected. Do not use needle if any imperfection is noted.
Note: After use, this product may be a potential biohazard. Handle and dispose of in accordance with accepted medical practice and with
applicable laws and regulations.
F. Precautions:
1. This product should be used by a physician who is completely familiar with the indications, contraindications, limitations, typical findings and
possible side effects of core needle biopsy, in particular, those relating to the specific organ being biopsied.
2. The introduction of the needle into the body should be carried out under imaging control (ultrasound, X-Ray, CT, etc.).
3. Never test the product by firing into the air. Damage may occur to the needle/cannula tip.
4. Unusual force applied to the stylet or unusual resistance against the stylet while extended out of the supportive cannula may cause the stylet to
bend at the specimen notch. A bent specimen notch may interfere with the needle function.
G. Potential Complications:
Potential complications of core biopsy are site specific and may consist of hematoma, hemorrhage, infection, adjacent tissue injury, and pain.
H. Equipment Required:
•
Appropriate imaging modality accessories
• Surgical gloves and drapes
• Local anasthetic
• Coaxial cannula (optional)
• Scalpel
• Sample collection container
• Other equipment as necessary
ENGLISH

 Loading...
Loading...