Precision™ Spinal Cord Stimulator System Clinician Manual
Clinician Manual
90607862-30 2 of 309
Device and Product
Description
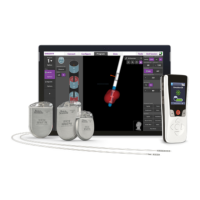
The Precision system consists of an
implantable pulse generator, temporary and
permanent percutaneous leads, surgical
paddle leads, and lead extensions, each
packaged as a separate kit. Single use
accessories and disposable tools are also
included in these kits.
Features of the Precision SCS System
include:
• Stimulation electrode eld navigation
• Sixteen independent current-controlled
electrodes
• Four programmable stimulation areas per
program; four possible
programs
• Long-life operation
• High-range parameter capability
• Small size
• Two-foot programming range
• This product contains no detectable latex
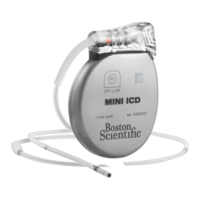
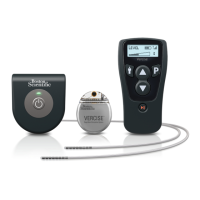
Implantable Pulse Generator
The Precision Implantable Pulse Generator
(IPG) system is intended to treat chronic
pain by electrically stimulating the spinal
cord. The multi-channel, multi-electrode
device capability provides exibility in
conjunction with ease of programming. A
rechargeable battery increases IPG longevity
and output capability while reducing size
and device replacement surgeries. The
IPG is controlled by a handheld Remote
Control, and can be engaged by a clinician
computer using proprietary Bionic Navigator
software. Periodically, the IPG battery
requires replenishing with an RF charging
device provided separately in the Patient
Charging Kit.
Leads
The percutaneous and surgical paddle
leads function as a component of the
Precision SCS system by delivering electrical
stimulation to the nerve structures in the
dorsal aspect of the spinal cord, resulting in
an inhibition of pain sensation.
Surgical Paddle Lead
The 2x8 Surgical Paddle Lead is available
in lengths of 50 cm and 70 cm. The distal
(paddle) end of the lead has two columns of
eight evenly spaced planar electrodes. Each
electrode is 3x2 mm
2
in area. On the proximal
side, this lead employs 2 lead tails. The end
of each tail has eight evenly spaced contacts,
identical to the percutaneous leads. The right
tail of the Paddle Lead is laser-etched to
allow for ease of right and left identication.
Each tail can be inserted into an IPG or into a
lead extension.
Percutaneous Leads
Percutaneous Leads are available in lengths
of 30 cm, 50 cm, and 70 cm. Each lead has
eight electrode contacts located near the
distal end. Each contact is 3 mm in length
and is spaced 1, 4 or 6 mm from the adjacent
contact. The Innion™ 16 percutaneous
lead is available in lengths of 50 cm and
70 cm. Each lead has 16 contacts located
near the distal end. Each contact is 3 mm in
length and is spaced 1 mm from the adjacent
contact. The 16 contact lead must be inserted
into a Splitter 2x8 which then connects to a

 Loading...
Loading...