Do you have a question about the Boston Scientific WaveWriter Alpha and is the answer not in the manual?
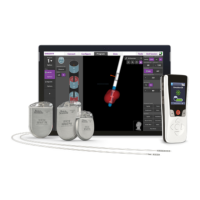
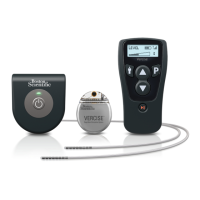
Key capabilities and design aspects of the WaveWriter Alpha and Prime Systems.
Conditions where the therapy is not recommended for patients.
Guidance and warnings for patients on device usage, charging, and potential issues.
Key procedures, warnings, and device interactions for medical professionals.
Provides information on electromagnetic emissions and their compliance.
Details electromagnetic immunity requirements and intended use environment.
| Device Type | Spinal Cord Stimulator |
|---|---|
| Manufacturer | Boston Scientific |
| Battery Type | Rechargeable |
| Indications | Chronic Pain Management |
| Waveform Options | Multiple waveforms available |
| MRI Conditional | Yes, with specific conditions |
| Programming | Wireless Programming |
| Battery Life | Up to 10 years |
| Lead Options | Multiple, including percutaneous and surgical leads |









 Loading...
Loading...