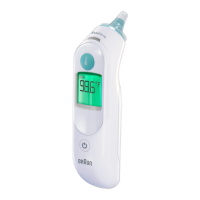
13
English
This infrared thermometer meets requirements established in ASTM Standard E 1965-98 (for the
thermometer system). Full responsibility for the conformance of the product to the standard is
assumed by Kaz USA, Inc., a Helen of Troy Company, 400 Donald Lynch Blvd., Suite 300,
Marlborough, MA 01752.
ASTM laboratory accuracy requirements for the thermometer only in the display range of 36 °C to
39°C (96.8 °F to 102.2 °F) for infrared thermometers is ±0.3 °C (±0.5 °F), whereas for mercury-in-glass
and electronic thermometers, the requirement per ASTM Standards E 667-86 and E 1112-86 is ±0.1
°C (±0.2 °F).
This device conforms to the following standards:
IEC 60601-1: Medical electrical equipment. General requirements for basic safety and
essential performance
ASTM E1965-98: Standard Specication for Infrared Thermometers for Intermittent Determination
of Patient Temperature
ISO 80601-2-56: Particular requirements for basic safety and essential performance of clinical
thermometers for body temperature measurement
IEC 60601-1-2: Medical electrical equipment – Part 1-2: General requirements for basic safety and
essential performance – Collateral Standard: Electromagnetic compatibility – Requirement and
tests
NOTE: Do not use this device in the presence of electromagnetic or other interference outside the
normal range specied in IEC 60601-1-2.
IEC 60601-1-11: Medical electrical equipment – Part 1-11: General requirements for basic safety
and essential performance – Collateral standard: Requirements for medical electrical equipment
and medical electrical systems used in the home healthcare environment
ISO 15223-1: Medical devices - Symbols to be used with medical device labels, labeling and
information to be supplied - Part 1: General requirements
ISO 10993-1: Biological evaluation of medical devices – Part 1: Evaluation and testing within a risk
management process
IEC 62304: Medical device software – Software lifecycle processes
ISO 14971: Medical devices - Application of risk management to medical devices
English

 Loading...
Loading...