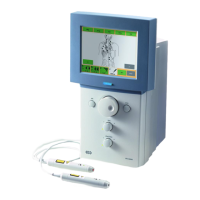
Do you have a question about the BTL 6000 SWT TOPLINE and is the answer not in the manual?
Details the two main components of the device: the main unit and the applicator.
Defines shockwaves, their properties, and therapeutic application parameters.
Explains shockwave generation methods, focusing on the ballistic principle.
Describes cellular and tissue-level effects, including pain relief mechanisms.
Highlights benefits like non-invasiveness, reduced pharmaceutical use, and non-surgical options.
Lists potential temporary side effects such as skin redness, swelling, or hematoma.
Lists various medical conditions and pain points suitable for shockwave therapy.
Details conditions or situations where shockwave therapy is not recommended.
Identifies and explains the function of controls and indicators on the device's front panel.
Illustrates and labels the components of the shockwave applicator.
Identifies and explains connectors, switches, and labels on the device's rear panel.
Step-by-step guide for unpacking, connecting power, and initial device setup.
Explains the initial screen, tab types, touch screen interface, and numeric keypad.
Covers setting therapy parameters via diagnosis, programs, manual input, intensity, and number of shocks.
Details how to start, pause, resume, and end a therapy session using device controls.
Guides on proper applicator contact and handling for effective shockwave application.
Instructions on saving custom therapy programs, diagnoses, and client data.
Manages client information, user-defined diagnoses/programs, and recent therapies.
Accesses menus for accessories, encyclopaedia, unit configurations, and specific settings.
Provides guidance on cleaning the exterior surfaces of the device and its parts.
Explains proper cleaning and disinfection of patient-contact accessories.
Details the procedure for safely replacing the device's fuse.
Instructs on how to empty and clean the condensate collection vessel.
Step-by-step guide for replacing the shock transmitter on the applicator.
Instructions for replacing worn internal parts of the applicator.
Covers essential safety measures for device operation, training, and patient contact.
Explains the meaning of various symbols used in the manual and on the device.
Outlines the warranty terms and conditions for the device and accessories.
Specifies environmental and operational parameters like temperature, humidity, and pressure.
Lists conditions for safe transport and storage of the device.
Details input power requirements, mains voltage, frequency, and protection class.
Provides physical specifications like weight, dimensions, and IP code.
Describes the touch screen and indicator lights on the device.
States the applied part type and class according to relevant directives.
Lists parameters that can be adjusted during therapy, like intensity and frequency.
Provides detailed technical specifications for the shockwaves generated.
Defines the step increments for adjusting intensity and frequency.
Lists technical specifications for the device's power supply adapter.
Details electromagnetic compatibility emissions and immunity test results.
Lists the various IEC, MDD, and EN ISO standards the device complies with.
Provides manufacturer contact details and information regarding product compliance.
| Technology | Shockwave Therapy |
|---|---|
| Frequency | 1-21 Hz |
| Display | 5.7" colour touch screen |
| Intensity Levels | Adjustable |
| Timer | Yes, adjustable |
| Pressure | Up to 5 bar |
| Presets | Yes |
| User Protocols | Yes |
| Pulse Modes | Continuous |
| Power Supply | 100-240 V, 50-60 Hz |