3
INDICATIONS
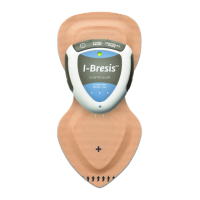
The I-Bresis™ System is indicated for the administration of soluble salts or other drugs into the body for medical purposes as an
alternative to hypodermic injection.
FOR ADDITIONAL IMPORTANT INFORMATION,
READ THE I-Bresis™ CONTROLLER AND CHARGING
STATION INSTRUCTIONS FOR USE.
CONTRAINDICATIONS
• Implanted Electronic Devices - Do not use this device on patients who have a cardiac pacemaker, implanted defibrillator,
or other implanted electronic device, because this may cause electric shock, burns, electrical interference, or death.
• Drug sensitivity – Do not use on patients with known sensitivity to the drug being administered.
• Pregnancy – Do not use on pregnant women. The safety of the system used during pregnancy has not been established.
• Scarring – Do not use on damaged skin, denuded skin or other recent scar tissue.
• Skin sensitivity – Do not use on patients with known sensitivity to electrical current or to the solution being administered.
• Head treatment – Do not treat across either the temporal region or the orbital region.
32
 Loading...
Loading...