The following symbols from the International Organization
for Standardization (ISO) and International Electrotechnical
Commission (IEC) are used throughout the product labeling
for the C
ONTOUR NEXT ONE blood glucose monitoring system
(meter packaging and labeling, and test strip and control
solution packaging and labeling).
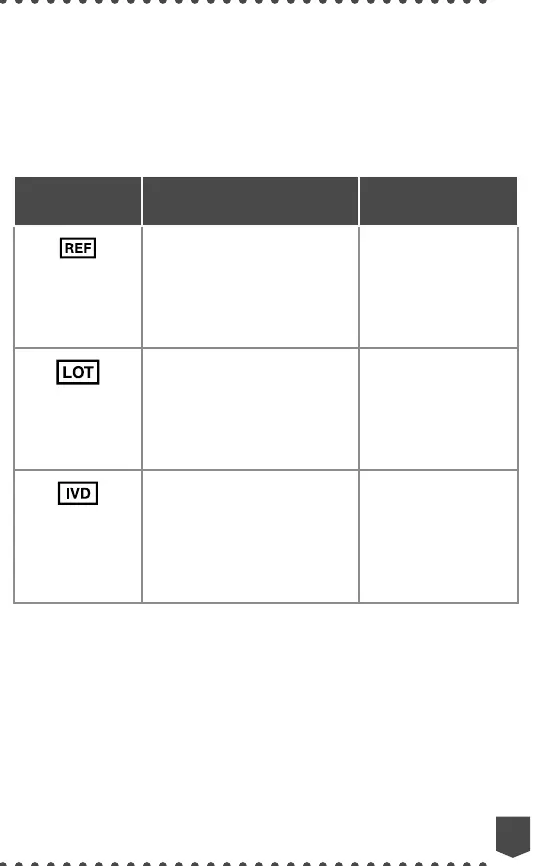
Symbol
Graphic/Title
Explanatory Text
Standard
Reference
6,7
Catalog
or model
number
Indicates the
manufacturer’s catalog
number so that the
medical device can be
¿º»ÄÊ¿Ű»ºƔ
ISO 15223-1,
Clause 5.1.6
Batch Code
Indicates the
manufacturer’s batch
code so that the medical
º»Ì¿¹»¹·Ä¸»¿º»ÄÊ¿Ű»ºƔ
ISO 15223-1,
Clause 5.1.5
IEC 61010-2-101,
Table 1,
Symbol 102
In Vitro
Diagnostic
Medical
Device
Indicates a medical
device that is intended
to be used as an in
vitro diagnostic medical
device.
ISO 15223-1,
Clause 5.5.1
60

 Loading...
Loading...