Page 16
Water Softening Process
The smallest units that make up chemical compounds and still retain the properties of those
compounds are called molecules. Molecules are made up of atoms or groups of atoms.
Electrically charged atoms are called ions. The charge of a single ion can be either positive
or negative. Ions of metals and minerals are usually positively charged, and are called cat-
ions. Ions such as chlorine, nitrate, phosphate, uoride, and sulfates are negatively charged,
and are called anions.
Certain insoluble materials are made up of large ions forming a skeletal structure contain-ing
oppositely charged ions. These ions can be exchanged with other similar ions in an ion ex-
change.
The rst commercial application of ion exchange was water softening in 1905. Since then, ion
exchange has been the most reliable and cost-effective method of softening and condition-
ing water in homes and industry.
The softening of water by ion exchange relies on the replacement of the calcium and mag-
nesium ions in the water by an equivalent number of sodium ions.
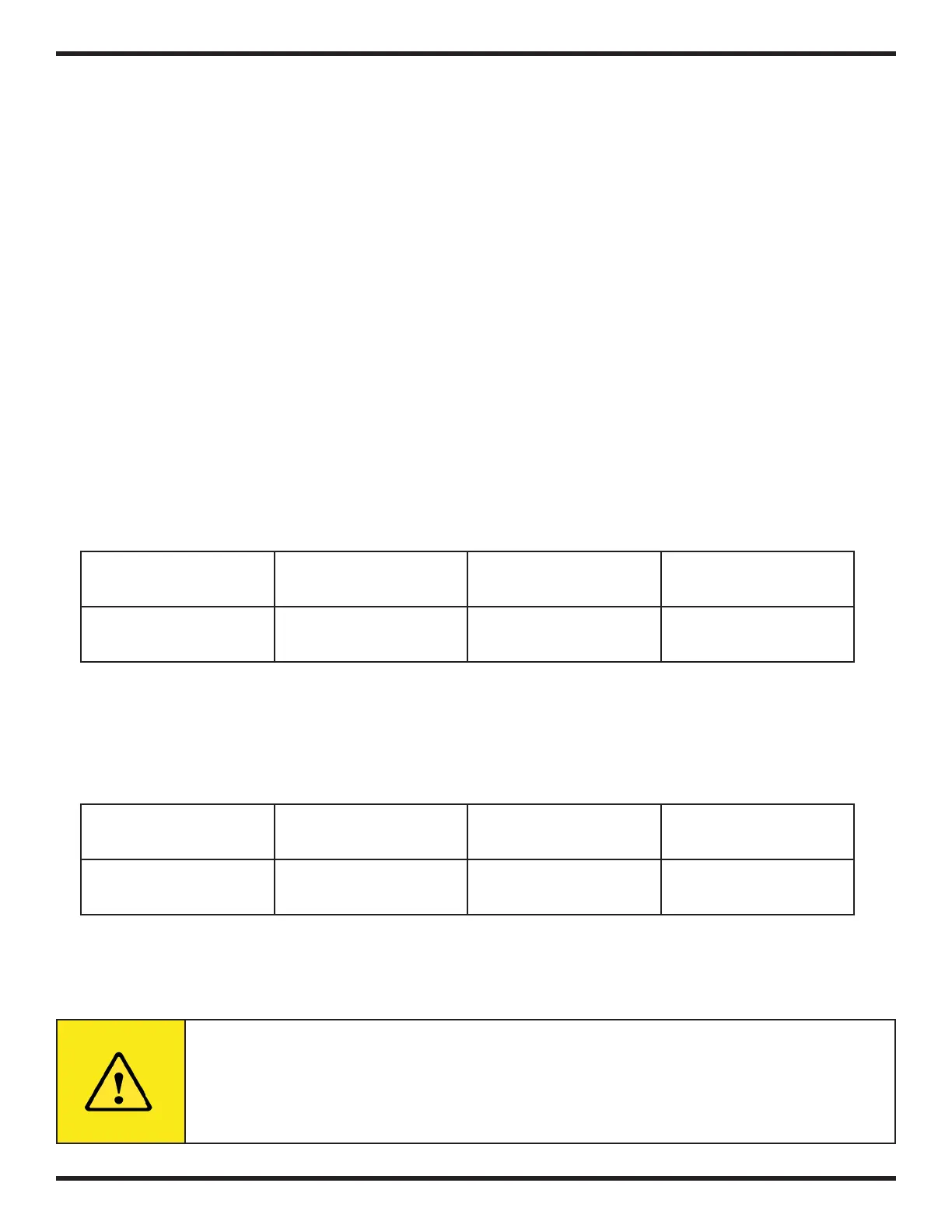
The softening process may be illustrated by the following equation:
R2.Na + Ca(HCO3)2 = R2.Ca+ 2NaHCO3
Sodium Ion Exchange
Resin
Calcium Bicarbonate in
Water
Calcium Ion Exchange
Resin
Sodium Bicarbonate in
Water
Obviously, the system can only exchange a certain amount of hardness and other contami-
nants before becoming exhausted. This is referred to as the capacity of the resin. The capac-
ity of the resin is referred to as grains of calcium carbonate hardness removed per cubic foot
of resin or Milliequivalents per liter. When the capacity has been exhausted, the resin needs
to be regenerated with a solution of sodium chloride (brine) as follows:
R2.Ca + 2NaCl = 2 R.Na + CaCl2
Calcium Ion Exchange
Resin
Sodium Chloride Brine
Sodium Ion Exchange
Resin
Calcium Chloride Wa-
ter
Ion exchange resins used in your Crusader Water Quality Management System are made
without harmful toxic solvents. This media is designed to be physically and chemically strong
while producing water that feels good, tastes great, and works hard for you.
Your Crusader Water Quality Management System can be regenerated with
Potassium Chloride salt if desired.
Crusader Pro, Deluxe, & Enhanced Systems Owner’s Manual 2020

 Loading...
Loading...