Do you have a question about the DENTSPLY Cavitron and is the answer not in the manual?
Lists approved applications for Cavitron inserts with ultrasonic scalers.
Specifies procedures where Cavitron inserts should not be used, like amalgam condensation.
Advises on discarding worn tips and handling inserts to prevent injury or contamination.
Emphasizes professional responsibility in understanding product use, patient health, and regulations.
States instruments are not sterile upon receipt and require sterilization before use.
Details steam autoclave sterilization cycles for bagged and non-bagged instruments.
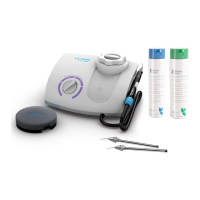
| Type | Ultrasonic Scaler |
|---|---|
| Power Source | Electric |
| Frequency | 25-30 kHz |
| Handpiece | Autoclavable |
| Power Output | Adjustable |
| Water Flow Rate | Adjustable |
| Weight | Varies by model |
| Dimensions | Varies by model |
| Power Supply | 110-230V AC, 50/60 Hz (depending on model and region) |
| Warranty | 1-2 years (varies by region) |







 Loading...
Loading...