General Ultrasound system requirements
Ultrasound System – Common Service Information 3-9
Direction 5444964-100 English Rev. 5
Electrical requirements for Console Ultrasound Systems (continued)
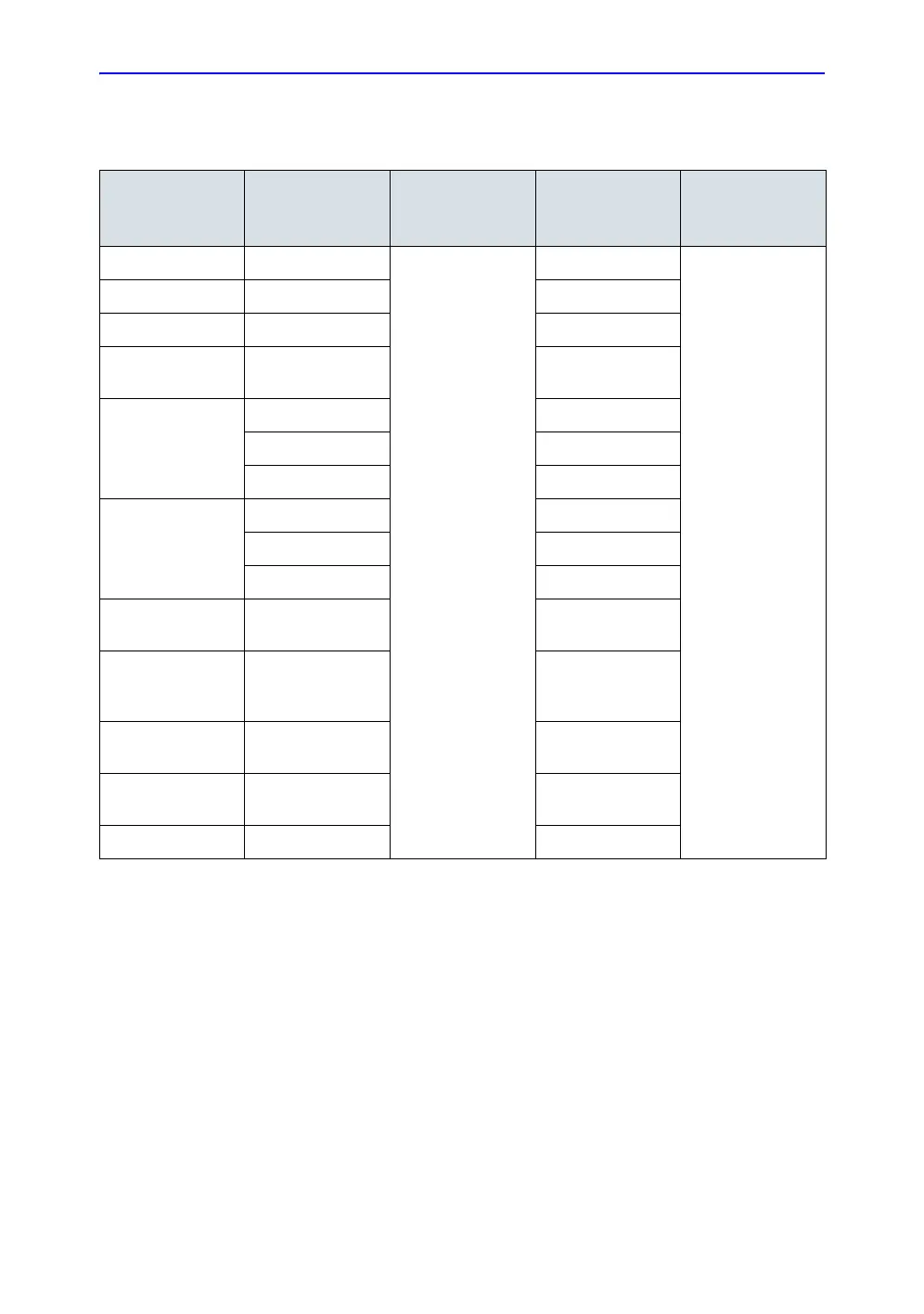
Table 3-5: Electrical Requirements - Vivid Consoles
Product Voltage Tolerance
Current or
Power
Consumption
Frequency
Vivid E7/E9 100-230 VAC
±10%
1100 W
50/60 Hz
Vivid 7 230 VAC 5 A
Vivid 7 100-120 VAC 10 A
Vivid S5/S5N
Vivid S6/S6N
100-240 VAC 0.5 to 1A
Vivid 4
100 VAC 8 A
120 VAC 8 A
220-240 VAC 4 A
Vivid 3
100 VAC 8 A
120 VAC 8 A
230 VAC 4 A
Vivid P3 100-120 VAC
220-240 VAC
425 VA
Vivid E95
Vivid E90
Vivid E80
100-240 VAC 700 W / 770 VA
Vivid S60/S70/
S60N/S70N
100-240 VAC 500VA
Vivid T8/Vivid T8
Pro
100-240 VAC 400VA
Vivid iq 100-240 VAC Max. 150VA
 Loading...
Loading...