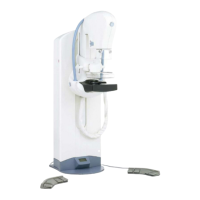
GE Healthcare Senographe 2000 D Acquisition System
REV 1 OM 5179217–1–100
1
REGULATORY REQUIREMENTS
This product complies with the regulatory requirements of the following:
- Council Directive 93/42/EEC concerning medical devices: the
0459
label affixed to the product
testifies compliance to the Directive.
For a system, the location of the CE marking label is described in the system manual.
European registered place of business:
GE Medical Systems Europe
Quality Assurance Manager
BP 34
F 78533 BUC CEDEX France
Tel: +33 (0)1 30 70 40 40
- Code of Federal Regulations Title 21, Subchapter J – Radiological Health.
- Underwriters’ Laboratories, Inc. (UL), an independent testing laboratory.
- Canadian Standards Association (CSA).
- International Electrotechnical Commission (IEC), international standards organization, when
applicable.
Compliance with these standards is evidenced by the presence of the appropriate labels on the
exterior of the Generator cabinet.
- USA/HHS:
United States Federal law restricts this device to use by or on the order of a
physician.
- General Electric Medical Systems is ISO 9001 and EN 46001 certified.
- The original document was written in English.
All components of the Senographe 2000 D system (Generator Cabinet, Gantry, Acquisition
WorkStation Cart) are designed to be suitable for use within the patient environment, and are
compliant with the relevant standards (UL 2601, IEC 601.1.1).
Note: Since the equipment allows the physician to store information on the patient with the function
IMAGE ANNOTATIONS, the European Directive regarding “the protection of the people with
regard of data management on their private life and to the free circulation of these data” requests
to the computerized file users (radiologists, physicians) not to store data related to their:
– race,
– philosophical opinions,
– religious opinions,
– political opinions,
– etc.
CAUTION
FOR TRAINING PURPOSES ONLY!
NOTE: Once downloaded, this document is UNCONTROLLED, and therefore may not be the latest revision. Always confirm revision status against a validated source (ie CDL).
 Loading...
Loading...