11
pp
pp
p
H CALIBRATIONH CALIBRATION
H CALIBRATIONH CALIBRATION
H CALIBRATION
Calibrate the instrument often, especially if high accuracy is required.
The instrument should be recalibrated :
a) Whenever the pH electrode is replaced.
b) At least once a week.
c) After testing aggressive chemicals.
d) After pressing RESET.
e) If higher accuracy is required.
PREPARATION
Pour small quantities of the buffer solutions into clean beakers. If
possible use plastic beakers to minimize any EMC interferences.
For accurate calibration and to minimize cross contamination, use two
beakers for each buffer solution. One for
rinsing the electrode and the second for
calibration.
If you are measuring in the acid range,
use pH 4.01 as second buffer; if you are measuring in the alkaline
range, use pH 10.01 or pH 9.18 as second buffer.
PROCEDURE
Calibration has a choice of 5 memorized buffers: pH 4.01, 6.86,
7.01, 9.18 and 10.01.
It is always recommended to perform a two-point calibration. The pH
meters however also provide for one-point calibration, as described
below.
HI 7007
HI 7004
HI 7004
HI 7007
CALIBRATION
HI 7007
RINSE
RESET
The RESET button (#14 on page 4;#15 on page 6) should only be
used when the instrument displays erroneous messages due to strong
electrical interference or when the instrument's power supply was
disconnected before the meter was switched off.
After pressing RESET always recalibrate the unit before proceeding.
22
Alkaline Error
1.0 Mol L
-1
Na
+
0.1 Mol L
-1
Na
+
Sodium Ion Correction for the Glass at 20-25°C
Concentration pH Error
13.00
13.50
14.00
12.50
13.00
13.50
14.00
0.10
0.14
0.20
0.10
0.18
0.29
0.40
TEMPERATURE CORRELATION FOR TEMPERATURE CORRELATION FOR
TEMPERATURE CORRELATION FOR TEMPERATURE CORRELATION FOR
TEMPERATURE CORRELATION FOR
pp
pp
p
HH
HH
H
SENSITIVE GLASSSENSITIVE GLASS
SENSITIVE GLASSSENSITIVE GLASS
SENSITIVE GLASS
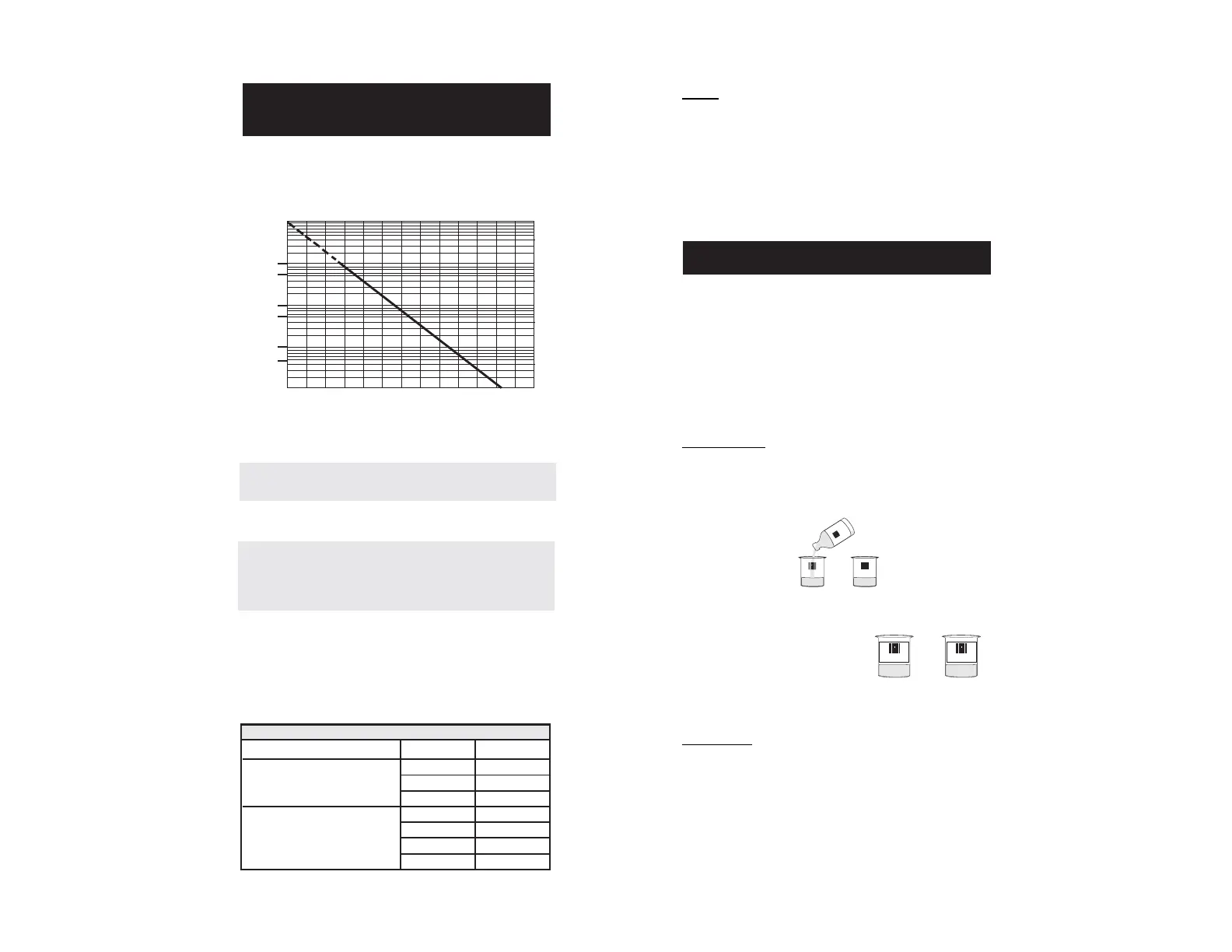
The resistance of glass electrodes partially depends on the temperature.
The lower the temperature, the higher the resistance. It takes longer time
for the reading to stabilize if the resistance is higher. In addition, the
response time will suffer to a greater degree at temperatures below 10C.
Since the resistance of the pH electrode is in the range of 200 Mohm,
the current across the membrane is in the pico Ampere range. Large
currents can disturb the calibration of the electrode for many hours.
For these reasons high humidity environments, short circuits and
static discharges are detrimental to a stable pH reading.
The pH electrode's life also depends on the temperature. If constantly
used at high temperatures, the electrode life is drastically reduced.
Typical Electrode Life
Ambient Temperature 1- 3 years
90 °C Less than 4 months
120°C Less than 1 month
High concentrations of sodium ions interfere with readings in alkaline
solutions; the pH at which the interference starts to be significant
depends upon the composition of the glass. This interference is the
alkaline error and causes the pH to be underestimated. Hanna's glass
formulations have the indicated characteristics.
-
-
-10
0 +10+20 +30+40 +50+60+70 +80+90
°
-10
-10
Ω
-10
-10
-10
-10
Ω
2x10
9
1x10
9
2x10
8
1x10
8
2x10
7
1x10
7
-20 -10 0 +10 +20+30+40 +50 +60 +70 +80 +90 C

 Loading...
Loading...











