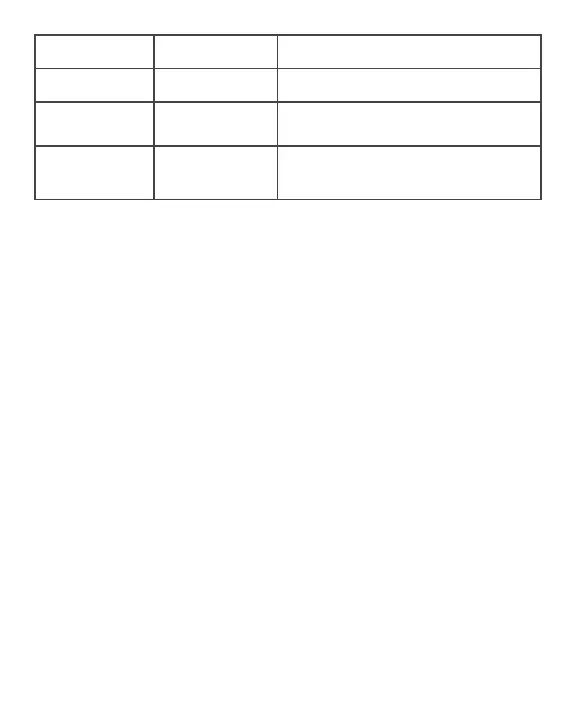
Air leak message:
Air Leak
Air leak Check for punctures in the wrap.
Low Battery Battery needs to be
charged
Plug in the control unit to charge the battery.
Cannot establish or
maintain a Bluetooth
connection
Bluetooth is turned off Turn on Bluetooth on the phone attempting to
pair with the Normatec Go control unit.
Devices not syncing
settings and/or
treatment
Devices are not paired. Check that the white pairing indicator light is illuminated
on both devices. If not, your devices are not paired.
Follow the “Using HyperSync™” instructions in the
Operating Instructions to pair your devices.
Call Hyperice customer service at +1.949.565.4994 if further assistance is needed.
WARRANTY
This product is covered by a limited warranty from Hyperice. Please visit hyperice.com/warranty to review the
warranty in your country.
FDA INFORMATION
MedWatch is the Food and Drug Administration’s (FDA) program for reporting serious reactions, product quality
problems, therapeutic inequivalence/failure, and product use errors with human medical products, including drugs,
biologic products, medical devices, dietary supplements, infant formula, and cosmetics. If you think you or someone
in your family has experienced a serious reaction to a medical product, you are encouraged to take the reporting
form to your doctor. Your health care provider can provide clinical information based on your medical record that can
help FDA evaluate your report. However, we understand that for a variety of reasons, you may not wish to have the
form filled out by health care provider, or your health care provider may choose not to complete the form. Your health
care provider is NOT required to report to the FDA. In these situations, you may complete the Online Reporting Form
yourself. You will receive an acknowledgment from FDA when your report is received. Reports are reviewed by FDA
staff. You will be personally contacted only if we need additional information.
SUBMITTING ADVERSE EVENT REPORTS TO FDA
Use one of the methods below to submit voluntary adverse event reports to the FDA:
a. Report online at:
www.accessdata.fda.gov/scripts/medwatch/index.cfm?action=reporting.home
b. Consumer Reporting Form FDA 3500B. Follow the instructions on the form to either fax or mail it in for
submission. For help filling out the form, see MedWatchLearn. The form is available at:
www.fda.gov/downloads/aboutFDA/reportsmanualsforms/forms/ucm349464.pdf
c. Call FDA at 1-800-FDA-1088 to report by telephone
Reporting Form FDA 3500 commonly used by heath professionals. The form is available at: www.fda.gov/downloads/
aboutFDA/reportmanualsforms/forms/ucm163919.pdf
 Loading...
Loading...