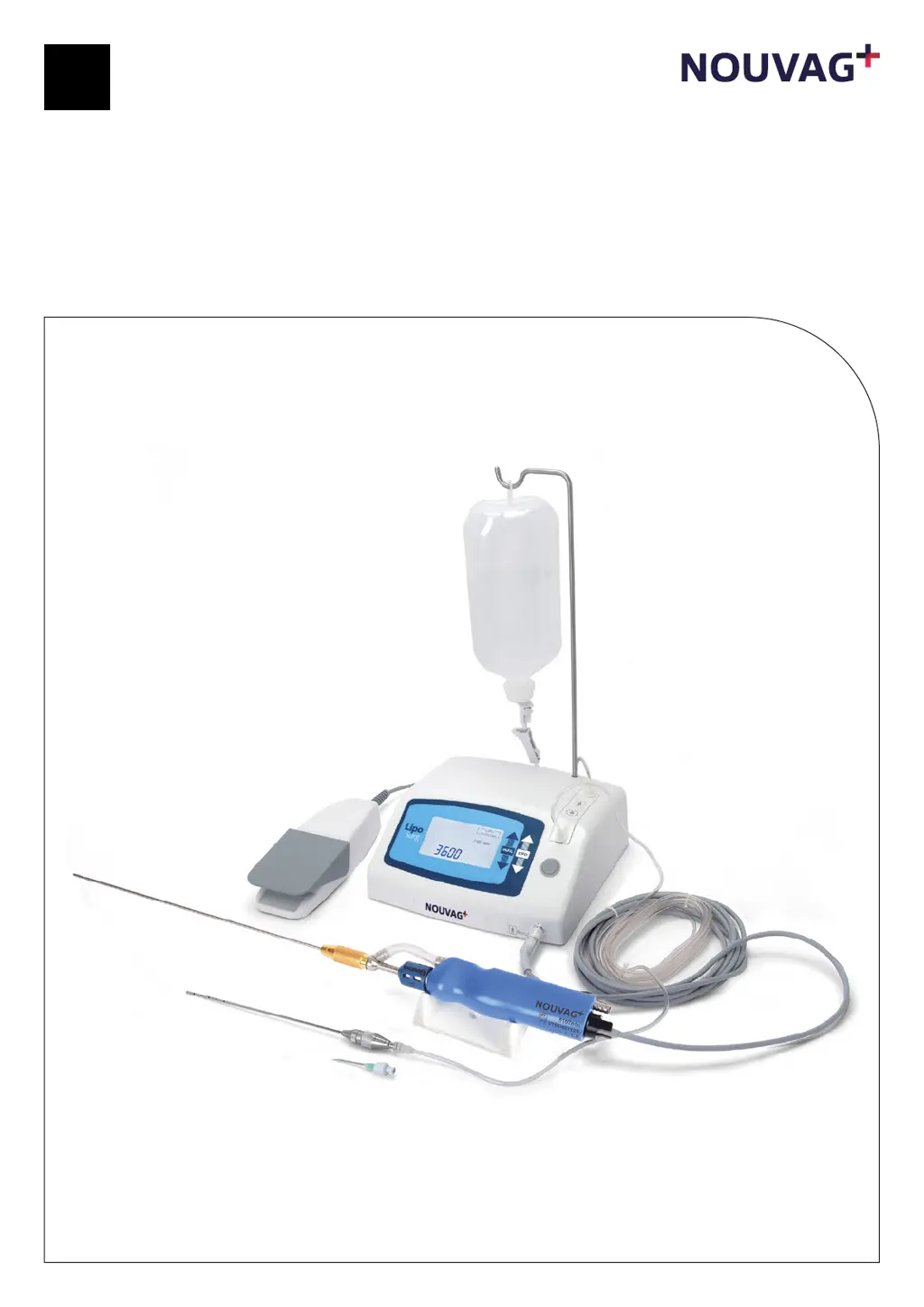
What to do if the Nouvag Medical Equipment pedal is not functional?
- AangelaedwardsAug 7, 2025
If the Nouvag Medical Equipment pedal isn't working, first ensure the pedal is properly connected to the socket at the back of the device. Also, verify that the control unit is switched on using the mains switch located at the back. If the problem persists, review the operating instructions to ensure correct operation.