2
Device Description
The Digitest® 3 Pulp Vitality Tester is a hand-
held, battery-powered dental diagnostic device
that identies a living tooth nerve by stimulating
it with a weak electric current. When the oper-
ator depresses the button, the strength of the
electrical stimulus automatically increases at
one of three preset rates. The unique waveform
is designed to trigger a patient response in a vital
nerve with a minimal amount of discomfort.
Intended Use/Indications
The Digitest 3 Pulp Vitality Tester is intended to
be used as a diagnostic instrument to assist in
the determination of the vitality of the dental pulp.
It is indicated for use on vital and non-vital adult
human teeth.
Contraindications
This Digitest 3 Pulp Vitality Tester is contrain-
dicated for use on a patient or by an operator
wearing a cardiac pacemaker or any other intra-
corporeal electronic device (internal debrillator,
insulin pump, etc.).
Warning
•
Do not modify this device. Modication may
violate safety codes, endanger the patient and
the operator, and void the warranty.
•
This device should only be used by licensed
dental professionals qualied in the use of
the unit.
• Read and understand all instruction manuals
before using the device.
• Portable RF communications equipment (in-
cluding peripherals such as antenna cables
and external antennas) should be used no
closer than 30 cm (12 inches) to any part of the
Digitest 3 unit, including cables specied by
the manufacturer. Otherwise, degradation of
the performance of this equipment may result.
• Use of this equipment adjacent to, or stacked
with other equipment, should be avoided be-
cause it may result in improper operation.
• This device is to be operated with Parkell ac-
cessories only. Use of accessories other than
those specied, or provided by Parkell Inc.,
may result in increased electromagnetic emis-
sions or decreased electromagnetic immuni-
ty of this equipment or improper operation.
•
Equipment not suitable for use in the presence
of ammable or explosive gases. Use of dental
nitrous oxide/oxygen analgesia is acceptable.
Conformance to Standards
•
Parkell’s quality system is certied to ISO
13485, and this device conforms with IEC
60601-1, IEC 60601-1-2, CAN/CSA-C22.2 No.
60601-1 and IEC 60601-2-40.
Cleaning and Infection Control
of the Digitest 3
•
DO NOT AUTOCLAVE THE DIGITEST 3 POWER
UNIT, AS THIS WILL CAUSE DAMAGE TO IT.
•
Autoclaving and disinfecting do not remove
accumulated debris. Before autoclaving or
disinfecting accessories: Rinse the acces-
sories under warm running water for 30 sec-
onds to remove any external or internal soil
or debris. Using a soft soapy cleaning brush
to assist in the cleaning, if necessary. Use
non-ammoniated detergent or dishwashing
soap. Do not use ammoniated cleansers or
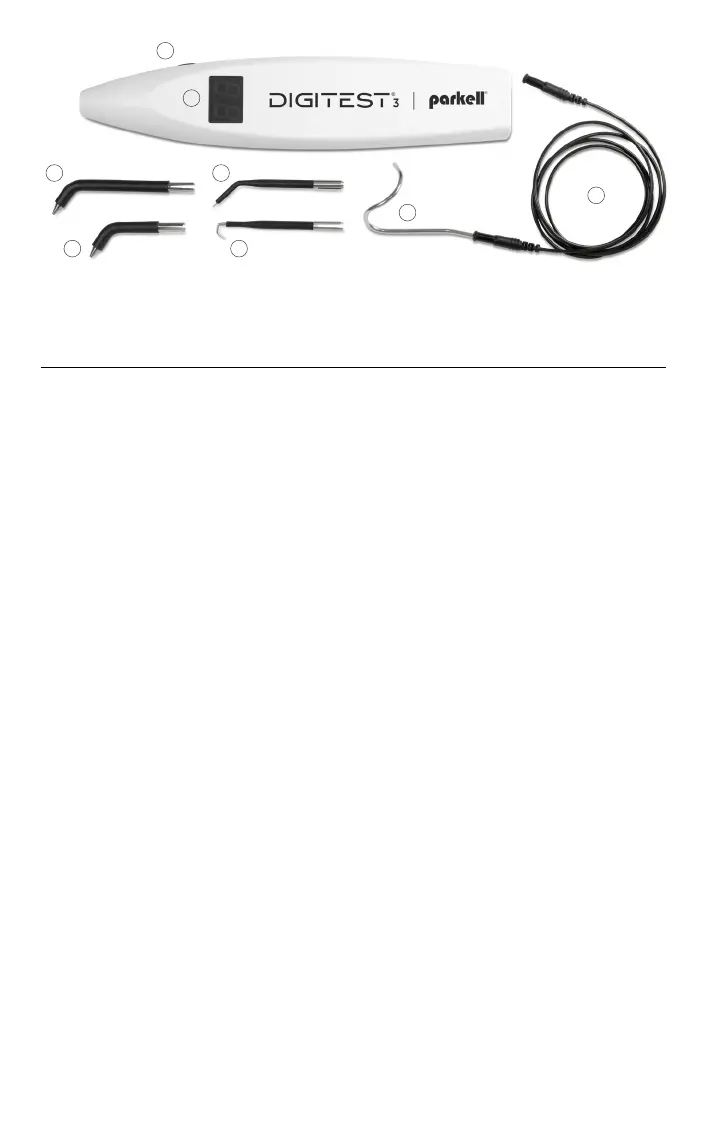
A. Stimulus Adjustment / Control Button
B. Digital Display
C. Ground Clip
D. Lead Wire
E. Posterior Autoclavable Probe
F. Anterior Autoclavable Probe
G. Precision Labial Autoclavable Probe
H. Precision Lingual Autoclavable Probe
A
B
C
D
E G
F H
 Loading...
Loading...