1 Safety Information
Safety Information
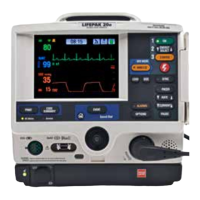
LIFEPAK 12 Defibrillator/Monitor Operating Instructions 1-3
© 2008-2010 Physio-Control, Inc.
Note: The LIFEPAK 12 defibrillator/monitor and its accessories that are intended for direct or
casual contact with the patient are latex-free.
SYMBOLS
The symbols below may be found in these Operating Instructions or on various configurations of the
LIFEPAK 12 defibrillator/monitor and accessories:
Possible electrical interference.
Using cables, electrodes, or accessories not specified for use with this device may result in increased
emissions or decreased resistance to electromagnetic interference which could affect the
performance of this device or of equipment in close proximity. Use only parts and accessories
specified in these Operating Instructions.
Possible device shutdown.
Always have immediate access to a spare, fully charged, properly maintained battery. Replace the
battery or connect the defibrillator to AC power when the device displays a low battery warning.
Possible improper device performance.
Using other manufacturers’ cables, electrodes, or batteries may cause the device to perform
improperly and invalidates the safety agency certification. Use only the accessories specified in these
Operating Instructions.
Possible improper device performance.
Changing factory default settings will change the behavior of the device. Changes to the default
settings must only be made by authorized personnel.
Possible failure to detect an out of range condition.
Reselecting QUICK SET will reset the alarm limits around the patient’s current vital sign values. This
may be outside the safe range for the patient.
Safety risk and possible equipment damage.
Monitors, defibrillators, and their accessories (including electrodes and cables) contain ferromagnetic
materials. As with all ferromagnetic equipment, these products must not be used in the presence of
the high magnetic field created by a Magnetic Resonance Imaging (MRI) device. The high magnetic
field created by an MRI device will attract the equipment with a force sufficient to cause death or
serious personal injury to persons between the equipment and the MRI device. This magnetic
attraction may also damage the equipment and affect the performance of the equipment. Skin burns
will also occur due to heating of electrically conductive materials such as patient leads and pulse
oximeter sensors. Consult the MRI manufacturer for more information.
CAUTION!
Possible device damage.
To help prevent component damage, do not mount device near vibration sources such as engine
struts and landing gear.
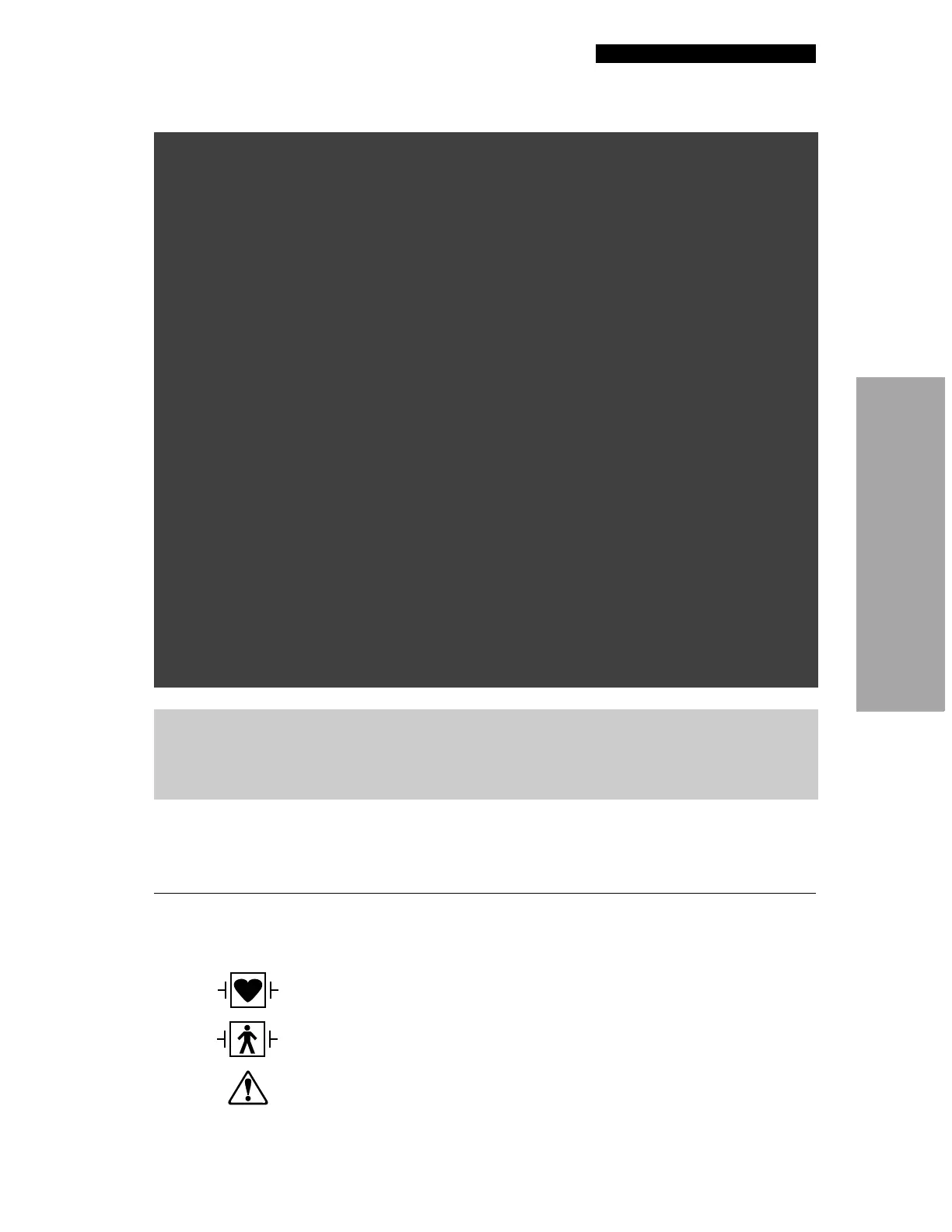
Defibrillation-proof type CF patient connection
Defibrillation protected, type BF patient connection
Attention, consult accompanying documents
 Loading...
Loading...