7
Maintenance, Inspection, and Testing
• Inspect the device on a continual basis. If a problem is
observed or suspected, the device should be returned
for repair.
• Inspect all components for cleanliness. If fluid or tissue
buildup is present, repeat the above cleaning and
disinfection procedures.
Packaging
N/A
Sterilization
After performing the cleaning instructions specified above, perform
one of the following sterilization cycles.
Steam
Note for United States users: For all autoclave-compatible devices,
Stryker recommends using steam sterilization instead of liquid
chemical sterilization.
• Steam sterilization is intended only for couplers marked
AUTOCLAVE.
• Rapid cooling, or “quenching,” the coupler after autoclaving
will result in product damage.
• The water used in the autoclave process must meet standards
for clean steam per AAMI ST 79 Appendix M – Steam Quality
requirements.
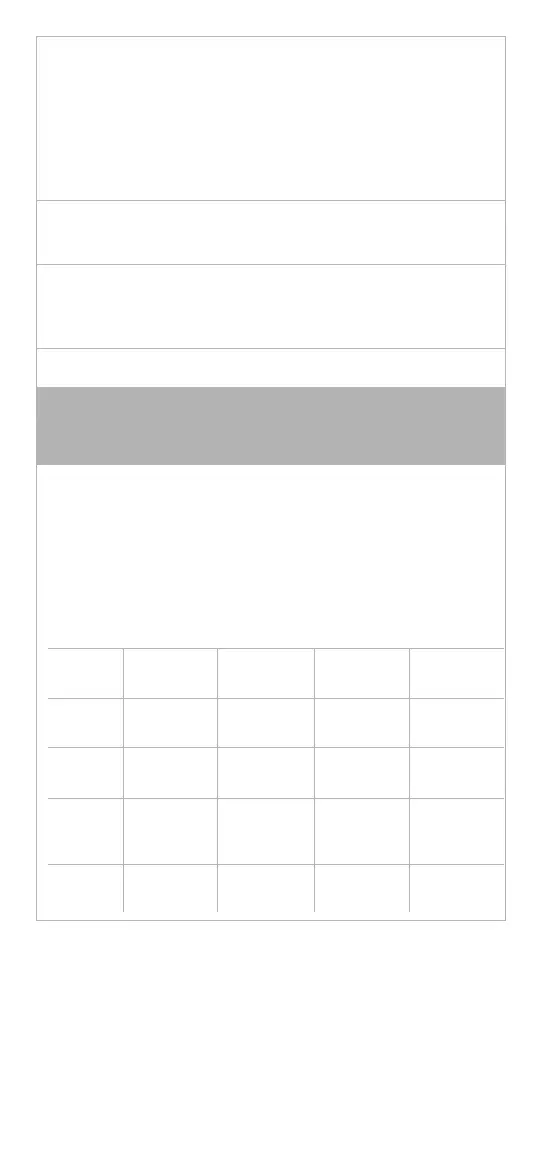
Sterilizer
Type
“Flash”
Gravity
“Flash”
Pre-vacuum
Gravity Pre-vacuum
Min.
Te m p.
132 – 137°C
(270 – 279°F)
132 – 137°C
(270 – 279°F)
132 – 137°C
(270 – 279°F)
132 – 137°C
(270 – 279°F)
Min. Cycle
Time
10 minutes 3 minutes 10 minutes 3 minutes
Product
Config-
uration
Unwrapped Unwrapped Double
wrapped
Double
wrapped
Drying
Time
— — 8 minutes 8 minutes

 Loading...
Loading...