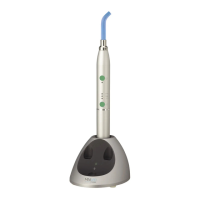
The SOPROLIFE is a Light Induced Fluorescence Evaluator designed to aid in the detection of caries and assist in treatment. It is a medical device manufactured by SOPRO, a company of ACTEON Group, and complies with European directive 93/42/EEC, as well as electrical safety and electromagnetic compatibility standards (IEC) (CEM).
Function Description
The SOPROLIFE system utilizes light-emitting diode (LED) lamps that emit blue light in diagnosis aid mode and treatment aid mode. This blue light causes healthy dentin to fluoresce with a green color. The device's optics and charged coupled device (CCD) sensor capture these fluorescence images, highlight them, and convert them into a video signal for display on a video monitor or computer monitor. This visual information aids dental practitioners in diagnosis and treatment planning.
In diagnosis aid mode (Mode I), the SOPROLIFE helps detect damage at various clinical stages with very high resolution. It allows for quick identification of areas for further examination using Gold Standard methods. Any color other than acid green, light green, or blue in the image should prompt the dental professional to examine that area using Gold Standard techniques. The device provides additional information to supplement visual observations, patient history, and other diagnostic techniques, but it does not provide a diagnosis itself.
In treatment aid mode (Mode II), the SOPROLIFE helps dental practitioners map unbroken tissue areas that are suspect. It assists in checking the quality of damaged tissue exeresis during or at the end of preparation in various clinical situations common in general practice.
The device also features a daylight mode which allows visualization of anatomical details invisible to the naked eye or with a mirror, enabling dentists to show "before" and "after" care differences.
Important Technical Specifications
SOPROLIFE Handpiece:
- High sensitivity CCD: 1/4”
- Resolution: (752 x 582) PAL; (768 x 494) NTSC
- Definition: 470 lines
- Sensitivity: 2 lux
- Lighting: Eight LEDs
- Adjustment: Four preset focusing positions (Extra-oral, Intra-oral, LIFE, Macro)
- Operating modes: 3 positions: DIAGNOSTIC aid mode, TREATMENT aid mode, and DAYLIGHT mode
- Image: Non-inverted
- Image capture: Through SoproTouch or footswitch (optional)
- Angle of view: 70°
- Cable length: 2.5m (optional 5m and 7m)
- Dimensions: L: 200 mm; W: 28 mm; H: 24 mm
- Usable part dimensions: W: 14.4 mm x d: 8 mm
- Weight: 78 g
- Applied part: BF-type
- Operating temperature: +10°C to +40°C
- Storage temperature: -20°C to +45°C
- Relative humidity: 10% to 90%
- Atmospheric pressure: 900 hPa to 1060 hPa
- Service: Continuous
- Water protection: Not protected against water chutes (IPX0)
- Flammable mixtures: Not adapted for use in the presence of an anaesthetic mixture flammable with air, oxygen, or dinitrogen monoxide.
Connection Boxes (Docks):
DOCK M_USB2 / DOCK M_VIDEO:
- Memory: One and four images
- Power supply: 115 V~ - 230 V~; 60 Hz - 50 Hz
- Video output: 1 PAL or NTSC video output, 1 PAL or NTSC S-video output
- Digital output: 1 digital USB output 2.0 (for DOCK M_USB2)
- Controller dimensions: L: 145 mm; W: 130 mm; H: 35 mm
- Controller weight: 245 g
DOCK U_USB2:
- Power supply: 24 V~; 50 Hz - 60 Hz
- Consumption: 15 VA
- Digital output: 1 digital USB output 2.0
- Controller dimensions: L: 50 mm; W: 75 mm; H: 36 mm
- Dock weight: 76 g
DOCK MU_USB2 / DOCK MU_VIDEO:
- Memory: One and four images
- Power supply: 24 V~; 50 Hz - 60 Hz
- Consumption: 10 VA
- Video output: 1 PAL or NTSC video output, 1 PAL or NTSC S-video output
- Digital output: 1 digital USB output 2.0 (for DOCK MU_USB2)
- Controller dimensions: L: 100 mm; W: 72 mm; H: 36 mm
- Dock weight: 190 g
DOCK USB2:
- Cable length: 3.5 m
- Digital output: 1 digital USB output 2.0
- Controller dimensions: L: 100 mm; l: 46 mm; H: 20 mm
- Dock weight: 165 g
- Powering: Directly through computer USB port (continuous 5 V low voltage type, 0.5 A).
Usage Features
- Focusing Adjustment: The handpiece features a rotating ring with four pre-set positions for easy focusing: Extra-oral (Portrait), Intra-Oral (1 to 5 teeth), LIFE (diseases observations), and Macro (details not visible to the naked eye).
- Image Freeze: Images can be frozen using SoproTouch on the handpiece or an optional footswitch.
- One-image mode: A slight touch on SoproTouch (or brief press of footswitch) freezes and stores the image. Another touch returns to direct mode or freezes a new image, deleting the previous one.
- Four-image mode: A slight touch on SoproTouch (or press of footswitch) stores an image in one quarter of the screen. Subsequent touches store additional images in other quarters until four images are obtained.
- Mode Selection:
- Button I: Switches between daylight mode and diagnosis aid mode.
- Button II: Switches between daylight mode and treatment aid mode.
- Alternating pushes on Button I then Button II switch between diagnosis aid mode and treatment aid mode.
- Accessories: The SOPROLIFE requires SOPROTIPS for use in diagnosis aid and treatment aid modes to displace ambient lighting. Dental barriers are also provided for intra-oral use to prevent cross-contamination.
- Computer Connectivity: The device can be connected to a computer via USB, requiring specific Windows® or MAC® configurations (detailed in the manual) and the SOPRO Imaging software for full functionality, including the LIFE function.
Maintenance Features
- General Maintenance: The camera does not require specific maintenance if used and cleaned according to manufacturer instructions. Complete disinfection is imperative before first use and after any servicing.
- Handpiece and Connection Box Disinfection:
- In case of contact with blood or excessive soiling, clean the handpiece with disinfecting wipes (e.g., Septol™ Wipes), then wrap it in several disinfecting wipes and leave for 15 minutes.
- For surface cleaning, use disinfecting wipes, remove excess moisture, and wipe the equipment until visibly clean. Allow to air dry.
- Precautions: Do not scrub, rinse, or immerse the handpiece or connection box in disinfecting liquid.
- Prohibited Chemicals: Do not use products containing ammoniac, trichloroethylene, dichloroethylene, ammonium hydrochlorid, chlorinated and aromatic hydrocarbon, ethylene dichloride, methylene chloride, or ketones, as these can deteriorate plastic parts.
- Do not directly spray disinfecting products on SOPRO products.
- SOPROTIPS Sterilization:
- SOPROTIPS must be clean before sterilization.
- They can be immersed in a disinfection bath and cleaned manually or with an ultrasonic cleaner.
- Rinse, dry, and package them before sterilization in an autoclave.
- Sterilization can be performed at 134°C and two bars (200 KPa) for 18 minutes.
- Note: Autoclave sterilization causes wear; it is recommended to replace SOPROTIPS every fifteen sterilization cycles to avoid distorted diagnoses.
- SOPROTIPS can also be cleaned with a disinfecting wipe (e.g., Septol Wipes).
- Infection Control: Strict infection control procedures must be observed when using SOPROTIPS and dental barriers to prevent cross-contamination between patients. If a hygienic protection is torn or the handpiece becomes "infected," thorough disinfection is essential.
- Troubleshooting: The manual provides a detailed troubleshooting guide for common issues related to video monitor and computer connections, image display, and clarity, along with recommended solutions.
- Returns for Repair: If the camera needs repair, it should be sent in its entirety (connection box, handpiece, cables) with a brief explanatory note of the defect. All defective parts must be sent for replacement. Upon return, the material should be checked for discrepancies, which must be confirmed by registered letter to the carrier within 48 hours.