7
TREATMENT SPACE
The ActivePure® Medical Guardian, model F170A is a device intended for medical purposes that is used in professional healthcare environments. The device
is designed to treat a space approximately 3,000 cubic feet that would have 8’ to 10’ ceilings. Recommended mode of action is to operate the device on the
highest speed possible continuously. The device recirculates ambient air continuously through the device where the lter using straining and attraction reduces
airborne contaminants from the air. Hydroxyl radicals and super ions created by the photo catalyst ActivePure® are mixed with the contaminant reduced air
which then enters the treatment space. The ActivePure® photo catalyst reduces the viability of microorganisms 4-6 log such as Staphylococcus epidermidis and
MS2 as tested, shown in Table 3. Larger spaces or operating on lower fan speeds may require multiple devices. Operational guidelines are provided to users in
the form of an Owner’s Manual. The length of time required to reach optimal reduction varies as a function of space volume and the dynamics of what occurs
within the treatment space including:
• Degree of contaminants in the air or being introduce by activities in the space
• Movement of people or equipment within the space
• Rates of ventilation
The device is intended to result in a 4-6 log reduction of airborne microorganisms from initial concentrations in 60 minutes as shown in Table 1. The ActivePure®
Medical Guardian, model F170A is not a sterile device and is not intended to create a sterile environment.
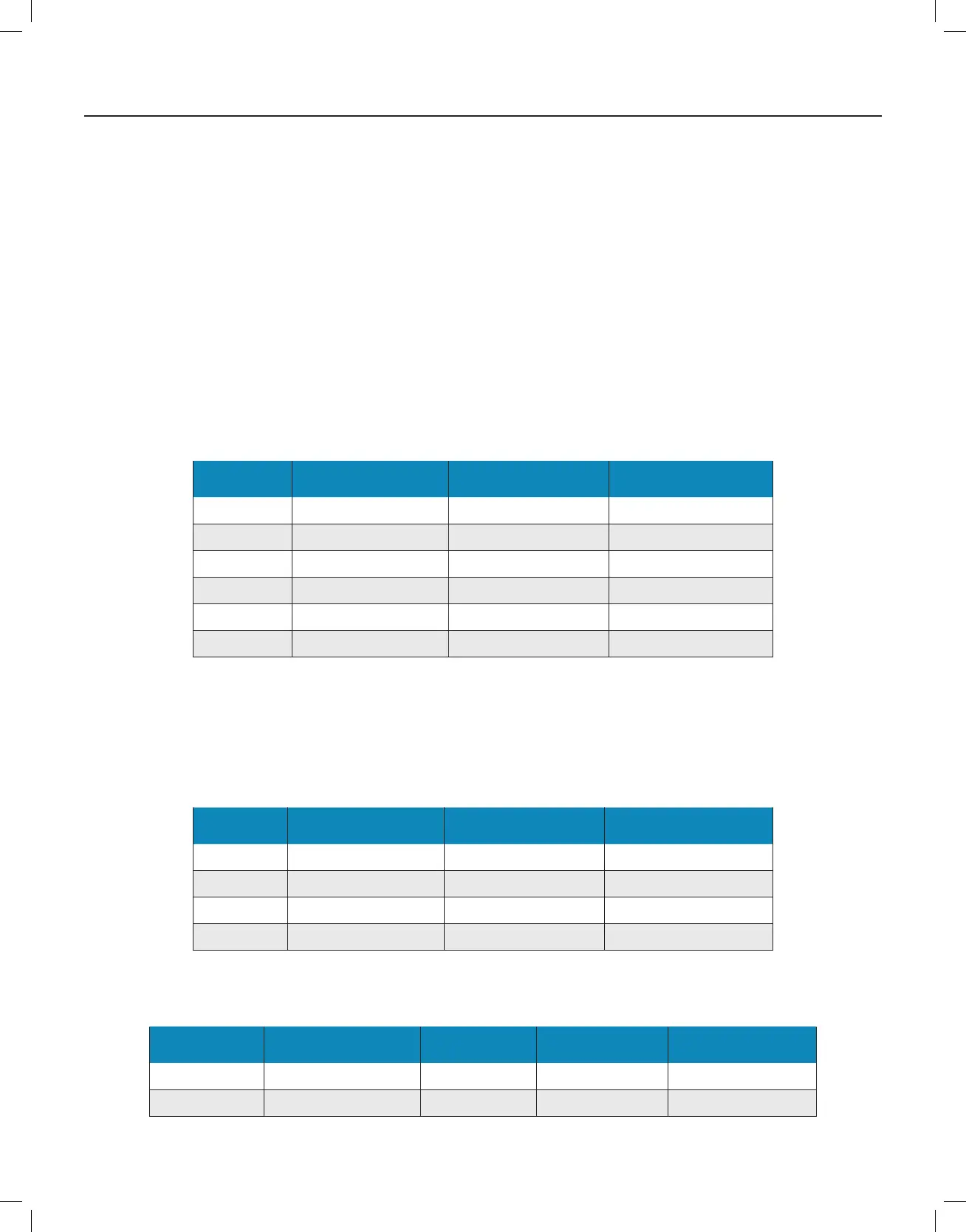
Organism
Type
Organism Name
Initial Load of
Microorganisms
Avg Log-Reduction after
60 minutes
Bacteria (gram +) Staphylococcus epidermidis 2.6e
5
cfu/L 5.95
Bacteria (gram -) Erwinia herbicola 1.9e
5
cfu/L 5.12
Virus (RNA) MS2 8.1e
5
cfu/L 5.58
Virus (DNA) Phi-X174 7.63e
3
cfu/L 4.19
Fungal spore Aspergillus niger 2.61e
4
cfu/L 4.12
Virus (DNA) Baillus globigii 7.95e
5
cfu/L 4.22
Fan Speed CFM of Air Movement
Cubic Feet Air
Movement in 1 Hour
Air Exchanges per Hour
based on 3,000 ft
3
Whisper 90 5,400 1.8
Low 100 6,000 2.0
Medium 180 10,800 3.6
High 300 18,000 6.0
Table 1
Table 2
Table 3
The information on the device CFM performance and air exchanges for the dierent speed settings is shown in Table 2, to illustrate the inuence of treatment
space to air exchanges on dierent fan speeds. These are provided to assist in determining the number of devices or fan speed settings necessary for a given space.
Organism Type Organism Name Test Temp / RH Exposure Time (H) Avg Log – Reduction
Bacteria, gram + Staphylococcus epidermidis 73.2°F / 50% 1 5.77
Virus, RNA MS2 bacteriophage 73.2°F / 50% 72 4.15
 Loading...
Loading...