The Advanced
®
Model 3250/4250 Service Manual
16
Regulatory Description
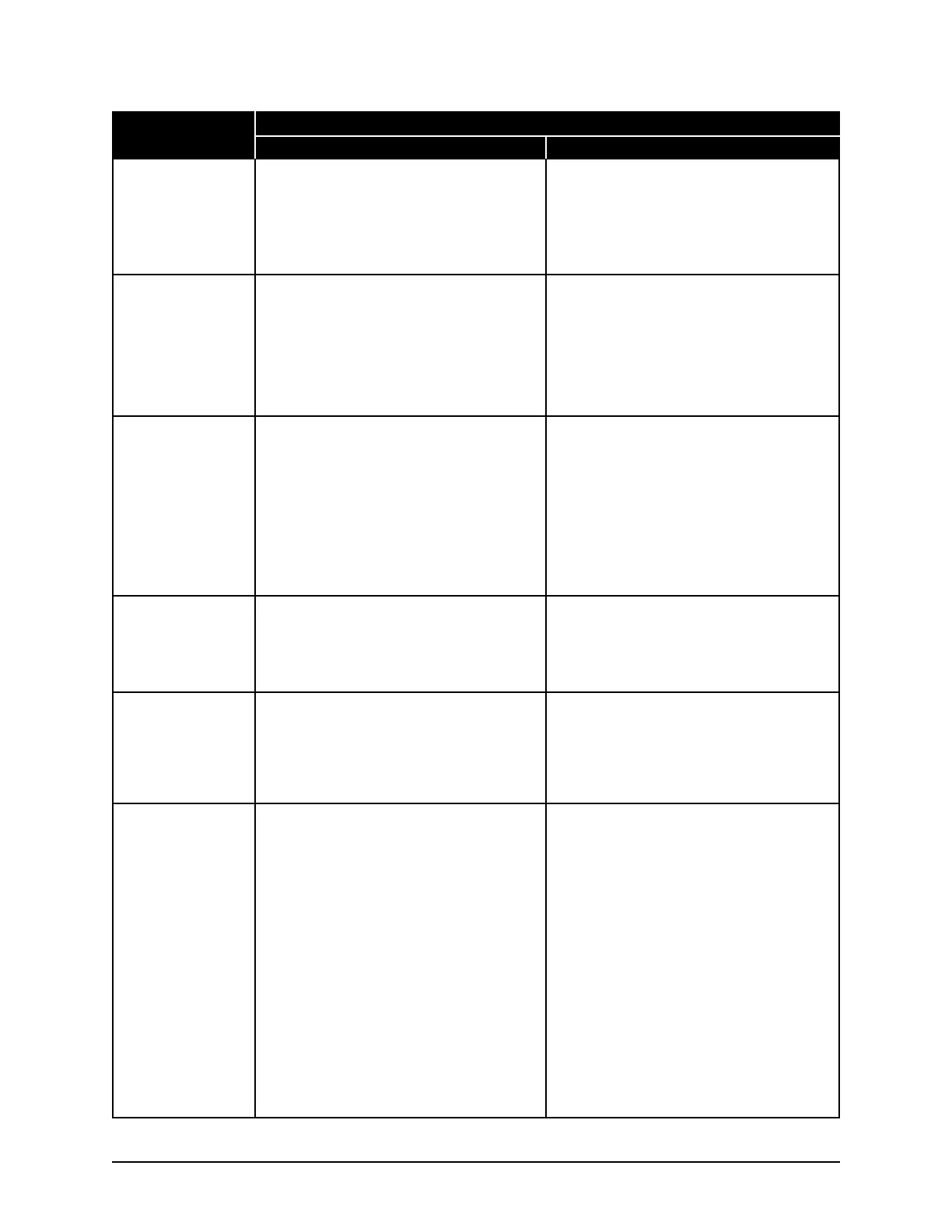
Approval Type Applies to Serial Suffix A - C Applies to Serial Suffix D and Higher
U.S. Safety
Canadian Safety
CE Declaration of
Conformity - EMC
CE Declaration of
Conformity - IVD
(3250 only)
CE Declaration of
Conformity - WEEE
CE Declaration of
Conformity - Low
Voltage
This product has been listed by ETL testing labo-
ratories as being in compliance with the require-
ments of UL 61010A-1, 1st Edition, "Electrical
Equipment for Laboratory Use; Part 1: General
Requirements". The "US" in the lower right of
the ETL mark demonstrates this listing.
This product has been listed by ETL testing labo-
ratories as being in compliance with the require-
ments of CAN/CSA C22.2 No.1010.1-92, "Safety
Requirements for Electrical Equipment for
Measurement, Control and Laboratory Use - Part
1: General Requirements"; Including Amendment
Two. The "C" in the lower left of the ETL mark
demonstrates this listing.
This product meets the intent of Directive
89/336/EEC for Electromagnetic Compatibility.
Compliance was demonstrated using the following
standards, as listed in the Official Journal of the
European Communities: Consult the Declaration
of Conformance certificate shipped with the prod-
uct for the latest update.
• EN 61326: 1997 with A1 & A2, Group 1, Class
B, "Electrical Equipment for Measurement,
Control, and Laboratory Use"
This product meets the intent of Directive
98/79/EC for In Vitro Diagnostic Medical
Devices. Consult the Declaration of Conformance
certificate shipped with the product (if required)
for the latest update.
This product meets the intent of Directive
2002/96/EC as amended by 2003/108/EC for
Waste Electrical and Electronic Equipment
(WEEE). Consult the Declaration of Conformance
certificate shipped with the product (if required)
for the latest update.
This product meets the intent of Directive
73/23/EEC, the Low Voltage Directive.
Compliance was demonstrated using the following
standards, as listed in the Official Journal of the
European Communities: Consult the Declaration
of Conformance certificate shipped with the prod-
uct (if required) for the latest update.
• EN 61010-1 (2001), “Safety Requirements for
Electrical Equipment for Measurement, Control,
and Laboratory Use - Part 1: General
Requirements”.
• EN 61010-2-101: 2002, “Safety Requirements
for Electrical Equipment for Measurement,
Control, and Laboratory Use - Part 2-101:
Particular Requirements for In Vitro Diagnostic
(IVD) Medical Equipment”.
This product has been listed by ETL testing labo-
ratories as being in compliance with the require-
ments of UL 61010-1, "Electrical Equipment for
Laboratory Use; Part 1: General Requirements".
The "US" in the lower right of the ETL mark
demonstrates this listing.
This product has been listed by ETL testing labo-
ratories as being in compliance with the require-
ments of CAN/CSA C22.2 No.61010.1, "Safety
Requirements for Electrical Equipment for
Measurement, Control and Laboratory Use - Part
1: General Requirements”. The "C" in the lower
left of the ETL mark demonstrates this listing.
This product meets the intent of Directive
2014/30/EU Conformity - EMC for Electromag-
netic Compatibility. Compliance was demonstrat-
ed using the following standards, as listed in the
Official Journal of the European Communities:
Consult the Declaration of Conformance certifi-
cate shipped with the product for the latest update.
• EN 61326-1, EN 55011, CISPR 11, Group 1,
Class B, "Electrical Equipment for
Measurement, Control, and Laboratory Use"
This product meets the intent of Directive
98/79/EC for In Vitro Diagnostic Medical
Devices. Consult the Declaration of Conformance
certificate shipped with the product (if required)
for the latest update.
This product meets the intent of Directive
2012/19/EU for Waste Electrical and Electronic
Equipment (WEEE). Consult the Declaration of
Conformance certificate shipped with the product
(if required) for the latest update.
This product meets the intent of Directive
2014/35/EU, the Low Voltage Directive.
Compliance was demonstrated using the follow-
ing standards, as listed in the Official Journal of
the European Communities: Consult the
Declaration of Conformance certificate shipped
with the product (if required) for the latest update.
• EN 61010-1, “Safety Requirements for
Electrical Equipment for Measurement, Control,
and Laboratory Use - Part 1: General
Requirements”.
• EN 61010-2-101, “Safety Requirements for
Electrical Equipment for Measurement, Control,
and Laboratory Use - Part 2-101: Particular
Requirements for In Vitro Diagnostic (IVD)
Medical Equipment”.
 Loading...
Loading...