12
4450A EN 20060221Introducing the CR 85-X
CR 85-X DIGITIZER
TÜV safety issues
Accessory equipment connected to the analog and digital interfaces must be
certified according to the respective IEC standards (e.g. IEC 950 for data
processing equipment and IEC 601-1 for medical equipment). Furthermore
all configurations shall comply with the valid version of the system standard
IEC 601-1-1. Everybody who connects additional equipment to the signal
input part or signal output part configures a medical system, and is therefore
responsible that the system complies with the requirements of the valid
version of the system standard IEC601-1-1. If in doubt, consult your local
service organization.
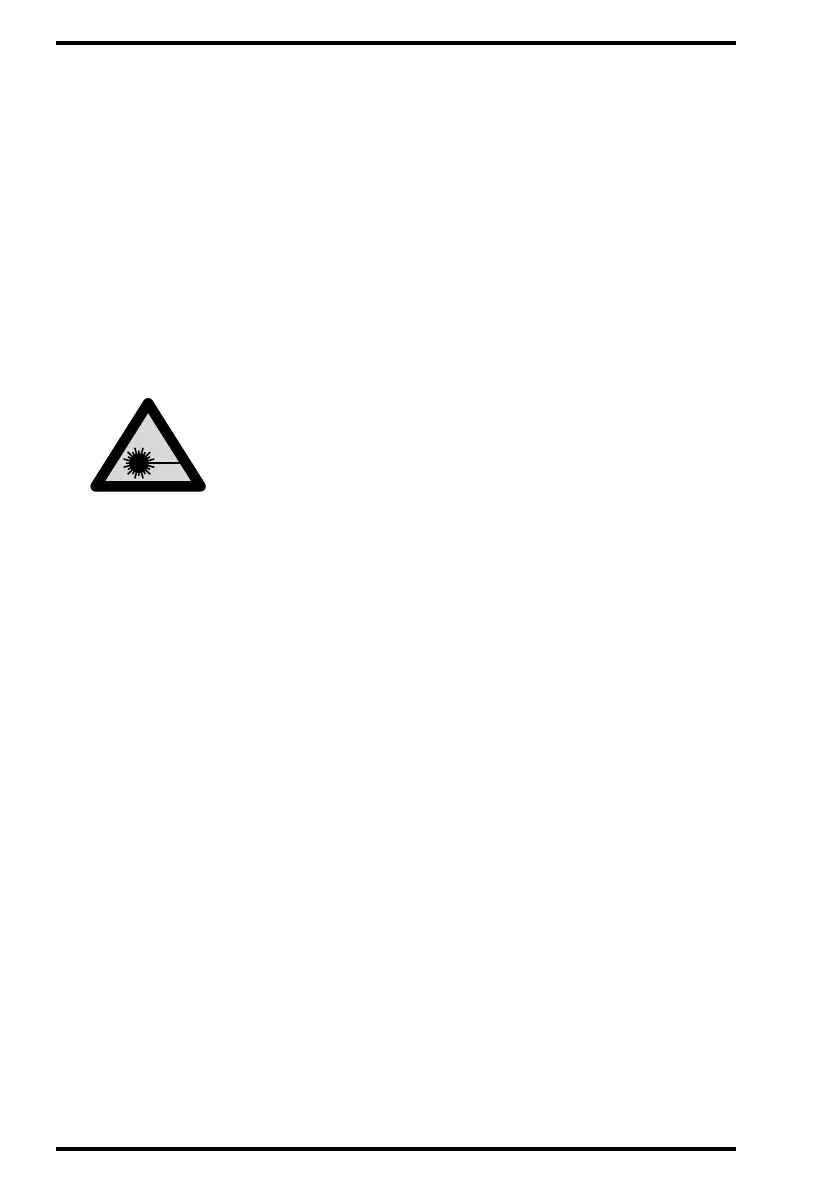
Safety instructions for laser products
The CR85-X is a Class 1 Laser Product. It uses a 80 mW laser diode,
classification class IIIb.
Under normal operating conditions - when both doors are closed - there can
be no laser radiation outside the CR 85-X. It is nonetheless imperative that the
local radiation safety regulations regarding the protection of staff against
scattered radiation are complied with, if the CR 85-X is located in the
immediate vicinity of an X-ray room.
Open the front left and right door only to solve cassette or image plate jams.
When you open either of the doors, the power supply of all critical
components is switched off automatically as a precaution.
 Loading...
Loading...