DW 953, 954, 955, 95FI
THE SALT CONTAINER
Rinses
Rinse 1 starts by flushing the water softener system for 40-90 seconds,
depending on setting, at the same time as the machine pumps out. This
is then followed by rinses as described in 1) and 2) above.
If hard water (i.e. containing lime) is allowed to dry on the dishes, glasses
etc., insoluble deposits of lime will be left.
Ca(HCO
3
)
2
CaCO
3
+ CO
2
+ H
2
O
Insoluble lime
If the water is softened with salt, sodium carbonate and calcium chloride
are formed. These two salts are water-soluble, and leave no deposits.
Ca(HCO
3
)
2
+ 2 NaCl = 2NaHCO
3
+ CaCl
2
2NaHCO
3
Na
2
CO
3
+ CO
2
+ H
2
O
Soluble soda
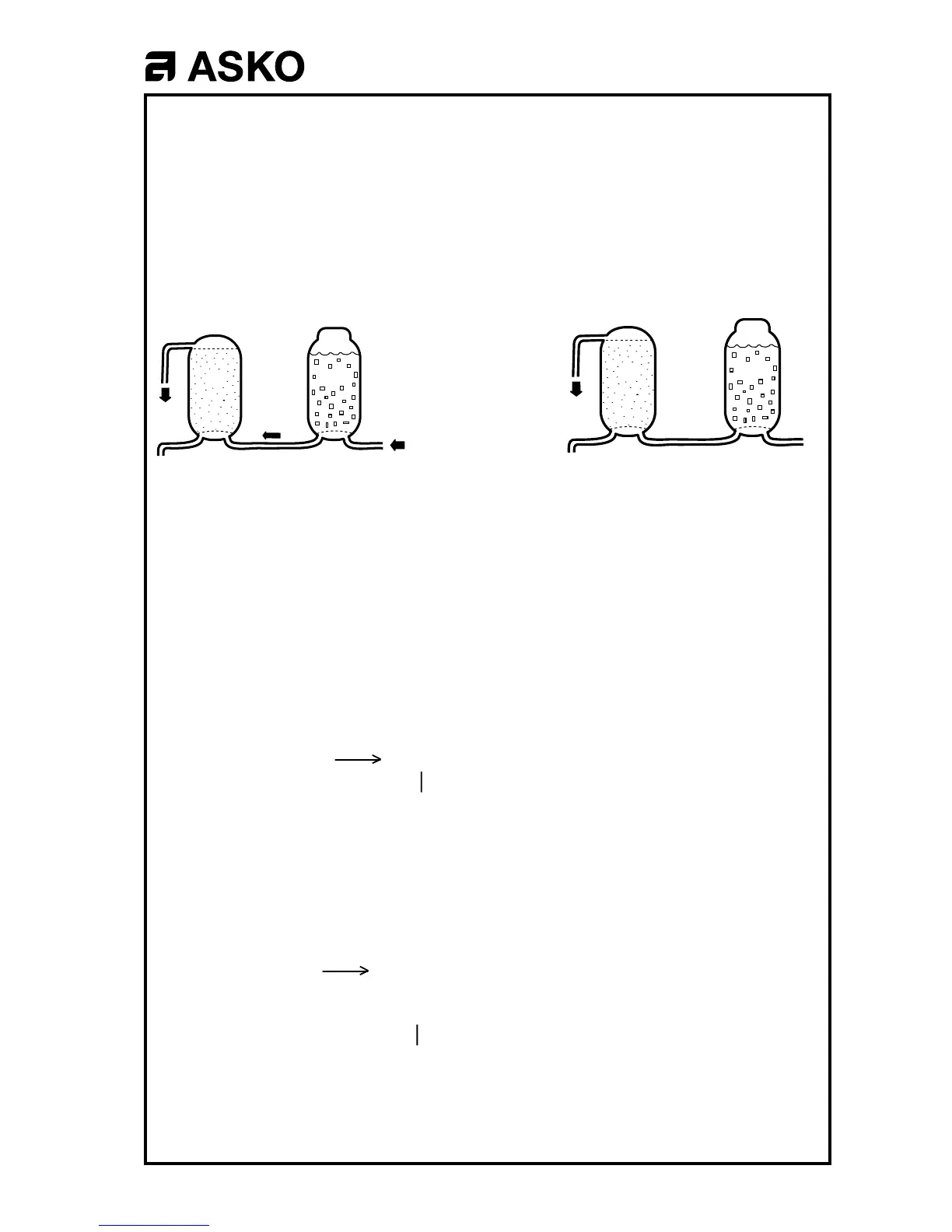
0,05 - 0,23
litres of water
flow into the
salt container,
where the Ca is
replaced by
Na. CaCl
2
is
flushed into the
machine.
Ca
2NaCl
Na Cl
Na
CaCl
Na Cl
2

 Loading...
Loading...