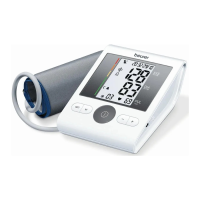
DE Oberarm-Blutdruckmessgerät
Gebrauchsanweisung ............4
EN Upper arm blood pressure
monitor
Instructions for use ..............25
FR Tensiomètre au bras
Mode d‘emploi ....................44
ES Tensiómetro de brazo
Manual de instrucciones ......64
IT Misuratore di pressione da
braccio
Istruzioni per l‘uso................84
TR Üst koldan tansiyon ölçme
cihazı
Kullanım kılavuzu ...............103
RU Прибор для измерения
кровяного давления в
плечевой артерии
Инструкция по
применению .....................121
PL Ciśnieniomierz naramienny
Instrukcja obsługi ...............142
NL Bloeddrukmeter voor de bove-
narm
Gebruiksaanwijzing ............162
DA Overarm-blodtryksmåler
Betjeningsvejledning ..........182
SV Blodtrycksmätare för överarm
Bruksanvisning ..................200
NO Blodtrykkmåler for overarm
Bruksanvisning ..................218
FI Verenpainemittari olkavarteen
Käyttöohje .........................236
BM 64