10
Problem/
Question
Possible Cause/Remedy
Why do I need
to replace the
atomiser at
regular inter
-
vals?
There are two reasons for this:
1. To guarantee a therapeutically
effective particle spectrum,
the nozzle holes should not
exceed a certain diameter.
Due to the thermal and me
-
chanical stresses, the plastic
is subjected to a certain
amount of wear. The nozzle
attachment [10] is particularly
susceptible to this wear. This
can also cause changes to
the droplet composition of the
aerosol, which has a direct ef-
fect on the effectiveness of the
treatment.
2. You are also recommended
to replace the atomiser on
a regular basis for hygiene
reasons.
Does each per
-
son need their
own atomiser?
This is absolutely necessary for
hygiene reasons.
11. Technical specifications
Model IH 21
Type IH 21/1
Dimensions
(WxHxD) 300 x 180 x 100 mm
Weight 1.65 kg
Operating
pressure approx. 0.8 - 1.45 bar
Atomiser fill
volume
max. 8 ml
min. 2 ml
Medicine flow approx. 0.3 ml/min
Sound pressure approx. 52 dBA
(acc. to DIN EN 13544-1 section 26)
Mains
connection
230 V~; 50 Hz; 230 VA
UK: 240 V~; 50 Hz; 240 VA
Saudi Arabia: 220 V~; 60 Hz; 220 VA
Expected service
life
1000 h
Operating
conditions
Temperature: +10 °C to +40 °C
Relative humidity: 10% to 95%
Atmospheric pressure: 700 to 1060 hPa
Storage and
transport
conditions
Temperature: 0 °C to +60 °C
Relative humidity: 10% to 95%
Atmospheric pressure: 500 to 1060 hPa
Aerosol
Properties
1) Flow: 5.3 l/min
2) Aerosol delivery: 0.326 ml
3)
Aerosol delivery rate: 0.132 ml/min
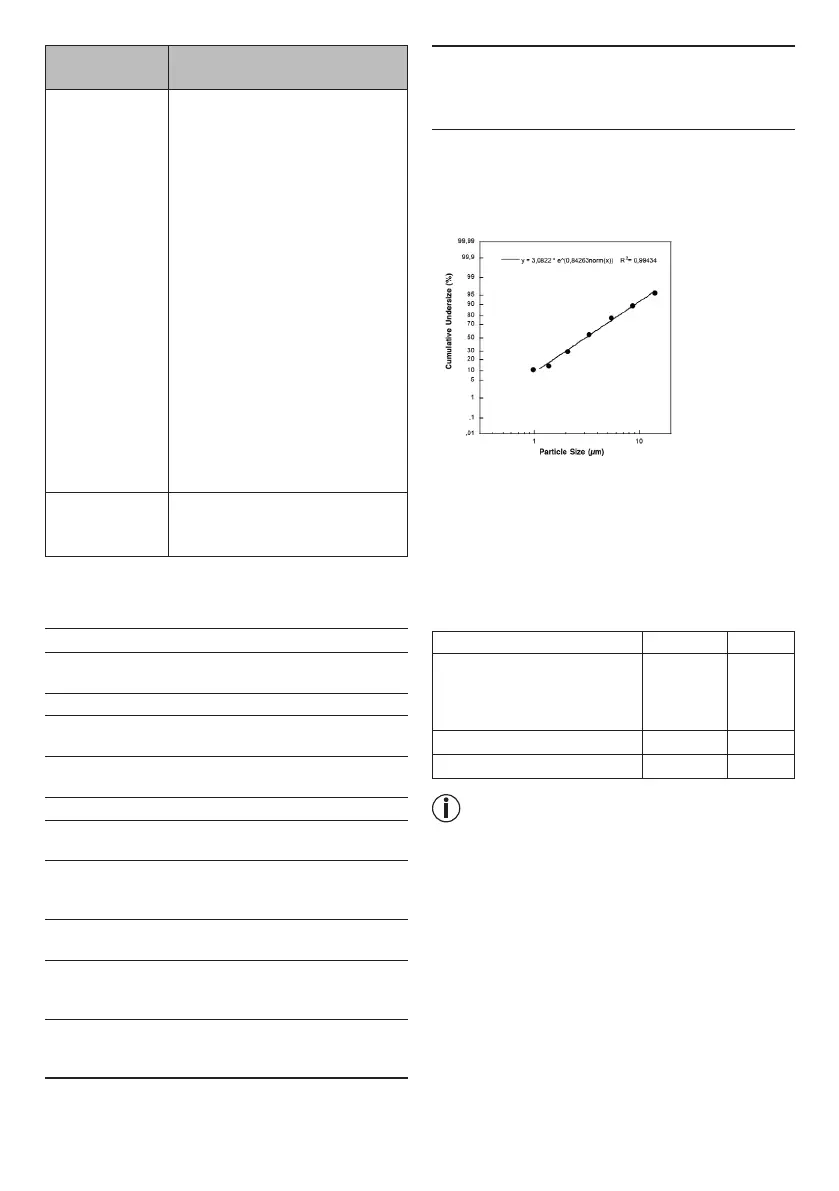
4) Particle size (MMAD): 3.07 µm
The serial number is located on the device or in the
battery compartment.
Subject to technical modifications.
Particle size diagram
Measurements were performed using a sodium fluo-
ride solution with a "Next Generation Impactor" (NGI).
This diagram may not be applicable for suspensions
or highly viscous medicines. More information can be
obtained from the relevant medicine manufacturer.
12.
Replacement parts and wearing
parts
Designation Material REF
Yearpack (contains Mouth
-
piece, Nosepiece, Adult mask,
Child mask, Atomiser, Com-
pressed air hose, Filter)
PP/ PVC 601.22
Nasal douche PP 601.37
Baby mask PVC 601.31
Note
If the unit is used outside of the specifications, proper
function is no longer guaranteed!
We reserve the right to make technical changes to im
-
prove and further develop the product.
This device and its accessories comply with Eu-
ropean standards EN60601-1 and EN60601-1-2
(CISPR 11, IEC61000-3-2, IEC61000-3-3, IEC61000-4-2,
IEC61000-4-3, IEC61000-4-4, EC61000-4-5,
IEC61000-4-6, IEC61000-4-7, IEC61000-4-8,
IEC61000-4-11), as well as EN13544-1, and is subject
to special safety measures in terms of electromagnetic
tolerance. The unit conforms to the requirements of the
European Directive for Medical Products 93/42/EEC, the
MPG (German Medical units Act).
 Loading...
Loading...