The Lambert-Beer’s law identifies the relation between sample concentration and the
intensity of transmitted light. Absorbances and % transmittances are defined by the following
equations:
% Transmittance (%T)
%T = I
t
/I
0
*100=10
-εcl
* 100
Absorbance or optical density (A or O.D.)
A = log
10
1/T = log
10
I
0
/I
t
= log
10
10
εcl
= εcl
Where I
0
= Intensity of incident light
I
t
= Intensity of transmitted light
ε = Molar extinction coefficient (liter/mole/cm)
c = Sample concentration (mol/liter)
l = Optical pathlength
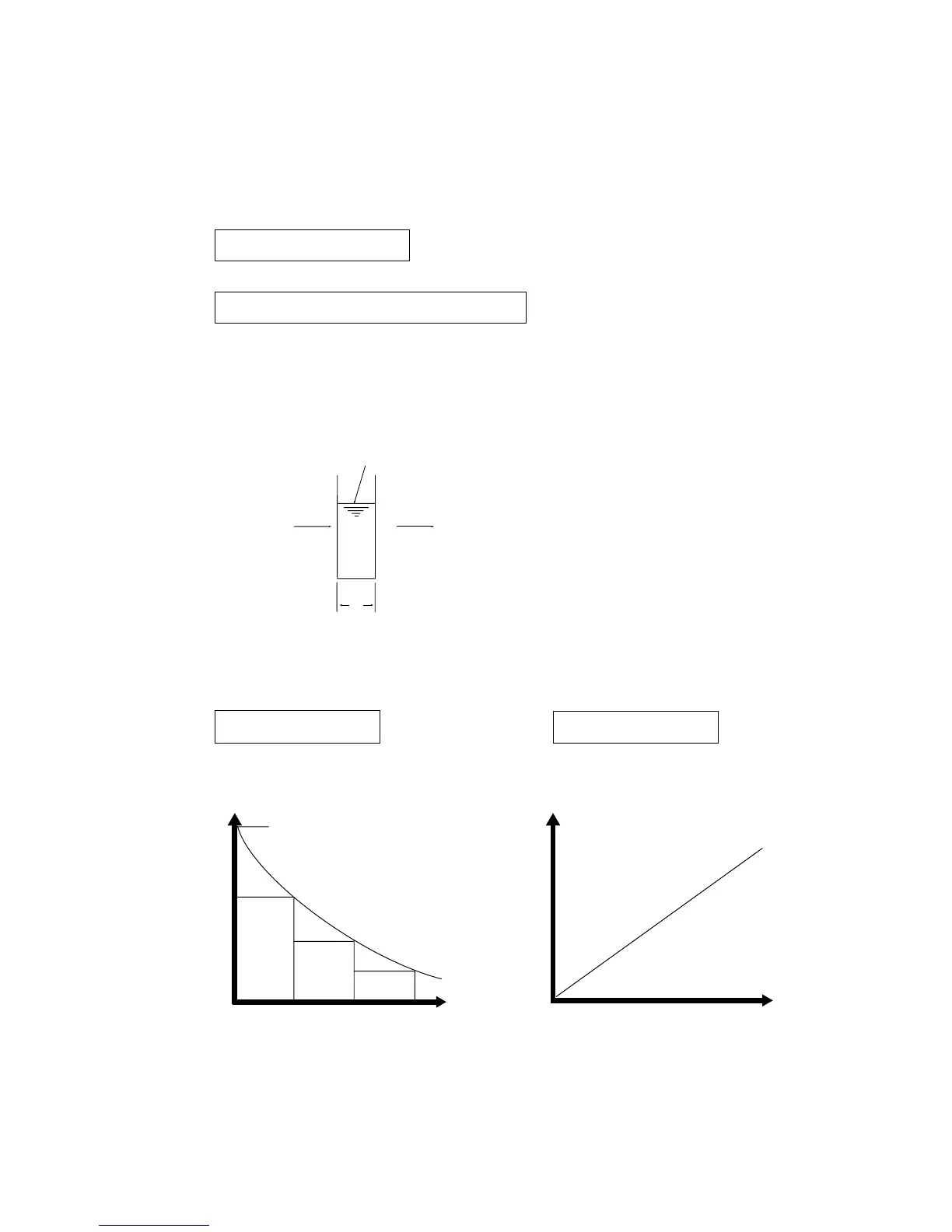
Fig. 6. Lambert-Beer's law.
A = log 1/T A = εcl
Fig. 7. Relationship between Transmittance and Concentration and Absorbance and
Concentration.
6
Transmittance
100
Absorbance
Concentration
Concentration
Sample concentration C
ll
ll
Incident light l
o
Transmitted light l
t
=l
o
10
-
εε
cll
ll
 Loading...
Loading...