Compu Chlor Manual AU 5
3.1.8 Check the expiration date of any chemical test kits as test results may be
inaccurate if used after that date.
3.1.9 When replacing the cell, only use replacement cells having a label that clearly
states that it is a replacement cell for this model.
4.0 THE CHEMISTRY INVOLVED
4.1.1 The Compu Chlor chlorine generator by electrolysis creates chlorine to sanitize
your pool using the salt molecules (NaCL) in your water. A small electric charge is
applied across a set of titanium plates inside the Electrolytic Cell. This produces
Sodium Hypochlorite (NaOCl). In water, Sodium Hypochlorite dissociates into
sodium (NA+) and hypochlorite (OCl-) ions.
4.1.2 It is the hypochlorite ions that form with the hydrogen (H+) ions (from the water) to
form hypochlorous acid (HOCl), which is the active agent that destroys bacteria and
algae, and oxidizes organic matter. This form of chlorine works quickly in the pipe,
leaving only a mild residual in the pool.
5.0 WATER CHEMISTRY
5.1 WARNING: Prior to turning on your Compu Chlor Chlorine
Generator for the first time your water chemistry must be balanced
according to the following guidelines.
5.2 Required Salt and Pool / Spa Chemistry Readings

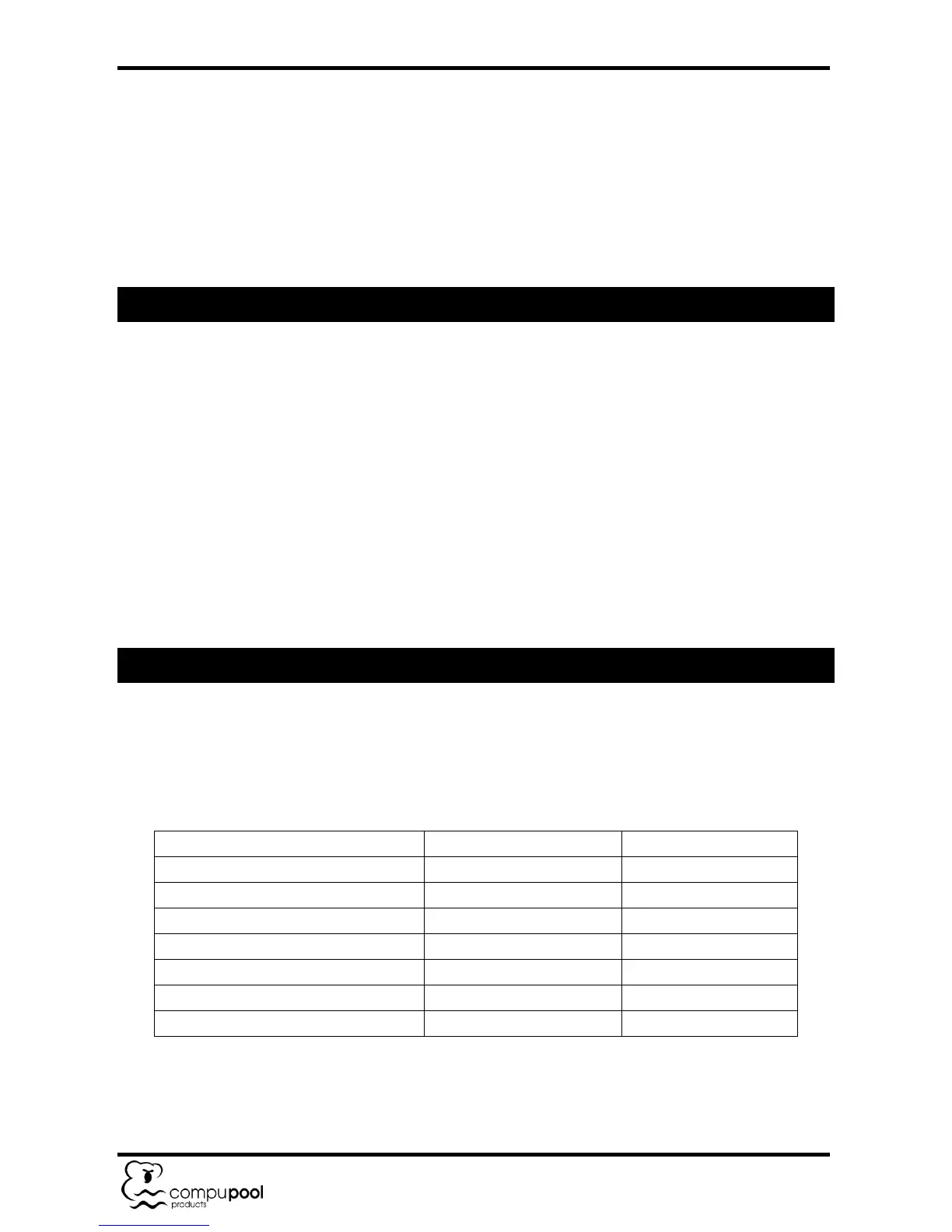 Loading...
Loading...