How does osmosis and reverse
osmosis work?
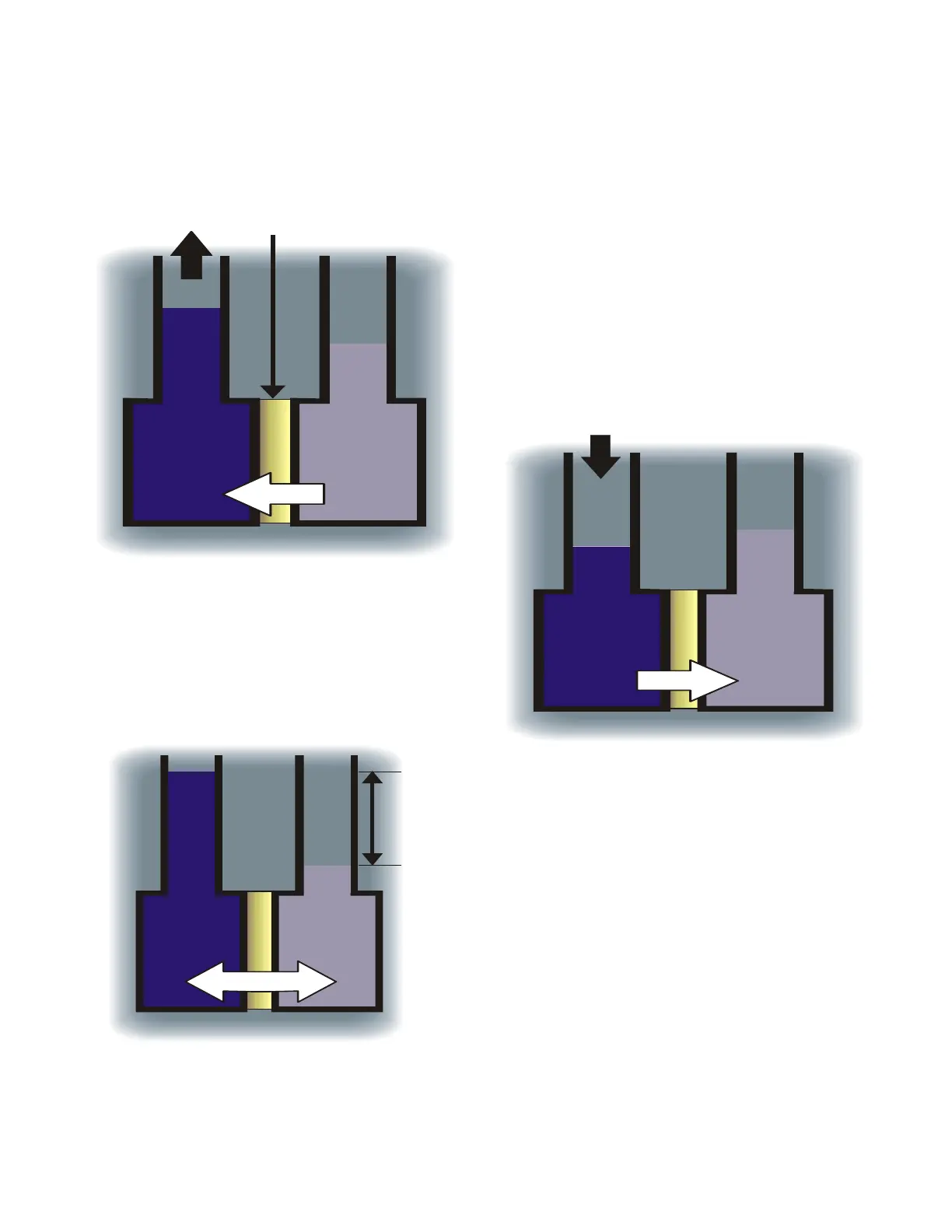
Fig. 1A: OSMOSIS
As shown in Figure 1A, under normal
pressure water will pass from the side
of the membrane with lower
concentration to the side with the
higher concentration to reach
equilibrium, below.
FIG. 1B: EQUILIBRIUM
Osmotic Pressure is the pressure
required to stop water flow and reach
equilibrium.
When the applied pressure equals
the osmotic pressure, the water flow
stops. When applied pressure
exceeds the osmotic pressure,
reverse osmosis will take place. In
reverse osmosis, water passes
through the membrane to the dilute
solution, leaving behind dissolved
particles. This process purifies the
water, often reducing total dissolved
solids content by 99%.
MEMBRANE
BRINE
WATER
FIG. 2A: REVERSE OSMOSIS
APPLIED PRESSURE
BRINE WATER
WATER
BRINE
Osmotic
Pressure
Crane Environmental systems use
semi-permeable spiral wound, thin
film membranes to separate and
remove dissolved solids, organic
material, pyrogens, submicron
colloidal matter, viruses, and bacteria
from water. Feed water is delivered
under pressure to the membranes,
where reverse osmosis takes place.
Water permeates the minute pores of
the membrane and is delivered as
purified product water. The
impurities in the water do not pass
through the membrane, and are
instead concentrated in the reject
stream that is flushed to the drain.
EPRO 150-8000 O&M MANUAL Rev.04/03 Page 3 of 55
 Loading...
Loading...