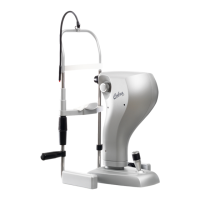
COBRA HD | IFUCOBRAHDEN00
INSTRUCTIONS FOR USE
This document is the property of
CSO Costruzione Strumenti Oftalmici srl.
Any reproduction, even partial, it is prohibited.
15/48
2.5 MEDICAL ELECTRICAL DEVICES CLASSIFICATION
Classification in compliance with the technical specification EN 60601-
1:2005 + A1:2012
Type of protection against the direct
and indirect contacts
Class II
Protection degree against humidity
IP20 (no protection against liquid
infiltration)
Sterilization or disinfection method
This device can be disinfected
Protection degree in presence of
anesthetics or inflammable
No protection
Electrical connection degree between
device and patient
Appliances with part applied on the
patient
2.6 ENVIRONMENTAL CONDITIONS
CAUTION
Danger of device damages. During transport and storage, the
device can be exposed to the environmental conditions for a
maximum period of 15 weeks, only if kept in the original packaging.
 Loading...
Loading...