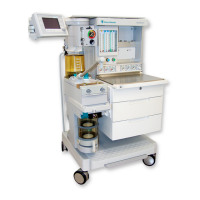
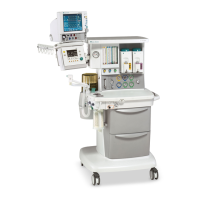
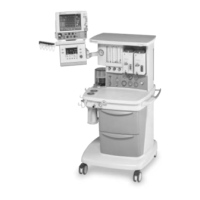
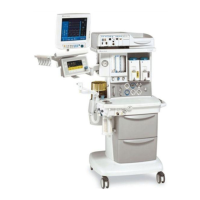
Conformity according to the Council Directive 93/42/EEC concerning Medical Devices
CAUTION: U.S. Federal law restricts this device to sale by or on the order of a licensed medical practitioner.
Outside the USA, check local laws for any restriction that may apply.
All specifications are subject to change without notice.
Document no. M1031869-01
August 2004
Datex-Ohmeda Inc. Datex-Ohmeda Division,
P.O. Box 7550 Instrumentarium Corporation
Madison, WI 53707-7550, USA P.O. Box 900
Tel: +1-608-221 1551 FI-00031 DATEX-OHMEDA, FINLAND
Fax: +1-608-222 9147 Tel: +358 10 39411
Fax: +358 9 1463310
www.us.datex-ohmeda.com
www.datex-ohmeda.com