1
Instructions for Use
For use in the Vena Cava
Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
A. Device Description
The DENALI
®
Vena Cava Filter is a venous interruption device designed to prevent pulmonary embolism. The
DENALI
®
Filter can be delivered via the femoral and jugular/subclavian approaches. A separate delivery system is
available for each approach. The DENALI
®
Filter is designed to act as a permanent filter. When clinically indicated,
the D
ENALI
®
Filter may be percutaneously removed after implantation according to the instructions provided under
the "Optional Procedure for Filter Removal" section.
The D
ENALI
®
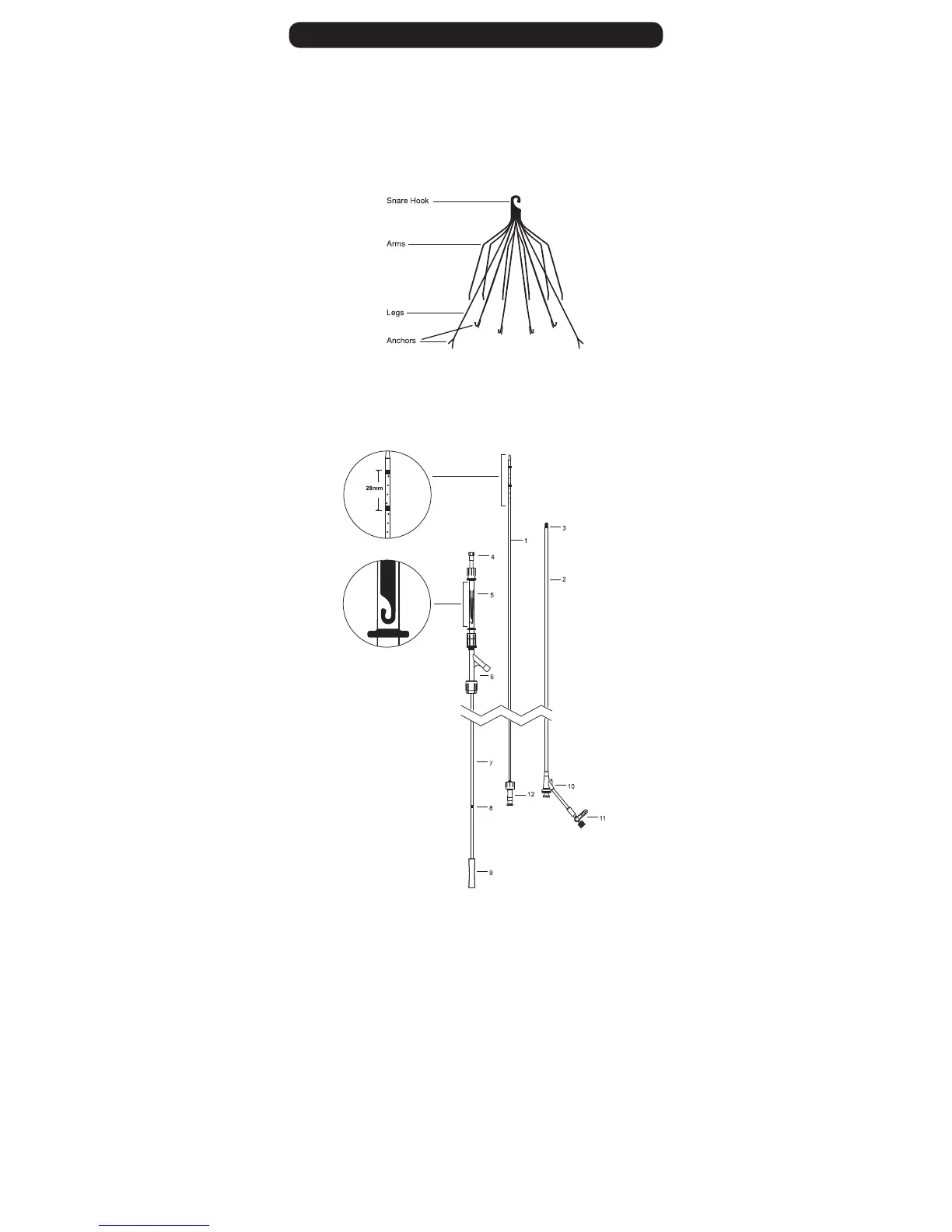
Filter consists of twelve shape-memory laser-cut nickel-titanium appendages. These twelve
appendages form two levels of filtration with the legs providing the lower level of filtration and the arms providing
the upper level of filtration. The DENALI
®
Filter is intended to be used in the inferior vena cava (IVC) with a diameter
less than or equal to 28mm.
Figure 1: DENALI
®
Filter (Supplied Preloaded)
The D
ENALI
®
Vena Cava Filter System consists of an introducer sheath and dilator, and a preloaded DENALI
®
Filter in
a storage tube with a pusher. The dilator accepts a 0.035" guidewire and allows for an 800 psi maximum pressure
contrast power injection. Radiopaque marker bands on the end of the dilator aid in measuring the maximum
indicated IVC diameter. They are spaced at a distance of 28mm (outer-to-outer). The 55cm, 8.4 French I.D.
introducer sheath contains a radiopaque marker and hemostasis valve with a side port. The pusher advances the
filter through the introducer sheath to the predeployment mark and is then used to fix the filter in place while the
filter is unsheathed. The DENALI
®
Vena Cava Filter Jugular/Subclavian System is illustrated in Figure 2.
Note: This product is not made with natural rubber latex.
Figure 2: D
ENALI
®
Vena Cava Filter Jugular/Subclavian System
B. MRI Safety:
The DENALI
®
Vena Cava Filter was determined to be MR-conditional according to the terminology specified in
the American Society for Testing and Materials (ASTM) International, Designation: F2503-05. Standard Practice
for Marking Medical Devices and Other Items for Safety in the Magnetic Resonance Environment. ASTM
International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, Pennsylvania, 2005.
Non-clinical testing demonstrated that the DENALI
®
Vena Cava Filter is MR Conditional. A patient with this implant
can be scanned safely immediately after placement under the following conditions:
• Static magnetic field of 3-Tesla or 1.5-Tesla
• Spatial gradient magnetic field of 720-Gauss/cm or less
• Maximum MR system reported whole-body-averaged specific absorption rate (SAR) of 2-W/kg in the normal
operating mode
In non-clinical testing, the D
ENALI
®
Vena Cava Filter produced a temperature rise of 2.7°C at a maximum MR
system-reported whole body averaged specific absorption rate (SAR) of 3-W/kg for 15-minutes of continuous MR
scanning in a 3-Tesla MR system using a transmit/receive body coil (Excite, Software G3.0-052B, General Electric
Healthcare, Milwaukee, WI).
MR image quality may be compromised if the area of interest is in the exact same area or relatively close to the
position of the D
ENALI
®
Vena Cava Filter. Therefore, optimization of MR imaging parameters to compensate for the
presence of this implant may be necessary.
Artifact Information:
Image artifact of the DENALI
®
Filter was tested according to ASTM F2119-07 in a GE HDx 3-Tesla cylindrical bore
scanner. The greatest artifact occurred at the snare hook and the ends of the arms and legs with the static field
parallel to the length. The maximum extent of the artifact beyond the metal of the phantom was 5mm for the spin
echo sequence and 10mm for the gradient echo sequence. Imaging parameters may need to be adjusted for
artifact optimization.
It is recommended that patients with a vena cava filter register the MR conditions with the MedicAlert Foundation (www.
medicalert.org).
C. Indications for Use
The DENALI
®
Filter is indicated for use in the prevention of recurrent pulmonary embolism via placement in the
vena cava in the following situations:
• Pulmonary thromboembolism when anticoagulants are contraindicated
• Failure of anticoagulant therapy for thromboembolic disease
• Emergency treatment following massive pulmonary embolism where anticipated benefits of conventional
therapy are reduced
ENGLISH
1 8F Dilator
2 Introducer Sheath
3 Introducer Radiopaque Marker Band
4 Safety Cap
5 Filter Storage Tube
6 Touhy-Borst Adapter
7 Pusher
8 Pre-Deployment Mark
9 Handle
10 Introducer Hub
11 Introducer Stop Cock
12 Dilator Hub
Note: Filter Snare Hook
should be in caudal orientation.
 Loading...
Loading...