4
Table 2: Primary Endpoints
Clinical Success of Placement (CSP) 95.0%
Technical Success of Placement (TSP) 99.5%
Technical Success of Retrieval (TSR) 97.6%
Clinical Success of Retrieval (CSR) 99.2%
There were no findings of filter fracture, cranial migration, caudal migration, filter tilt at placement, or filter tilt at
retrieval. Through the six month time point there were five (5) cases of symptomatic PE. Through the 24 month
time point there was one additional case of symptomatic PE. One case of PE led to a patient death. The patient
was considered active disease with DVT and PE noted at baseline and a contraindication to anticoagulation prior
to surgery. The site medical examiner listed the primary cause of death as pulmonary embolism and the secondary
cause of death as metastatic adenocarcinoma. The independent Clinical Events Committee (CEC) adjudicated
that the death was possibly related to the device. Through the six month time point there were five (5) cases of
asymptomatic penetration; none of which had clinical sequelae. Three (3) cases of penetration were noted at
implant and two (2) cases of penetration were noted at retrieval. Most instances of reported penetration were just
over the threshold measurement of 3mm outside of the cava wall, with penetrations reported 0.3, 0.6, 0.7, 1.3 and
3.6 mm beyond the threshold. Penetration was determined by digital subtraction venography at placement and
retrieval and was adjudicated by an independent core laboratory. Through the six month time point there were 20
patients that reported new or worsening DVT. Through the 24 month time point there were 6 additional patients that
reported new or worsening DVT. All new DVTs reported were in those patients that had active disease at the time
of implant, were considered to be hypercoagulable, suffered multi-trauma injuries, or those that had orthopedic
procedures on their lower extremities. All site-reported adverse events were adjudicated by the CEC and imaging
was analyzed by the Core Lab.
Table 3: Complication Rates
0-6 Months
1
0-24 Months
2
Recurrent PE
5 / 188 (2.7%) 6 / 200 (3.0%)
Caval Occlusion
1 / 188 (0%) 1 / 200 (0.5%)
New DVT
14 / 188 (10.1%) 18 / 200 (9.0%)
Worsening DVT
6 / 188 (7.4%) 8 / 200 (4.0%)
Filter Fracture
0 / 186 (0%) 0 / 186 (0%)
Cranial Migration > 2cm
0 / 186 (0%) 0 / 186 (0%)
Caudal Migration > 2cm
0 / 186 (0%) 0 / 186 (0%)
Filter Penetration at Placement > 3mm
3 / 200 (1.5%) 3 / 200 (1.5%)
Filter Penetration at Retrieval > 3mm
2 / 82 (2.4%) 2 / 124 (1.6%)
Filter Tilt at Placement > 15°
0 / 200 (0%) 0 / 200 (0%)
Filter Tilt at Retrieval > 15°
0 / 82 (0%) 0 / 124 (0%)
1
The 0-6 month time frame includes patients that completed the 6 month visit or had their filter retrieved within the 6 month window.
2
The 0-24 month time frame includes all patients that reported an event regardless of length of follow-up
DENALI
®
Filter Retrieval
DENALI
®
Filter retrieval was attempted in 124 patients and successful in 121 patients (97.6%). In the three (3)
unsuccessful retrieval cases, the snare was unable to engage the filter retrieval hook due to anatomical curvature
in two cases, and the filter was unable to be removed due to thrombus in the filter in one case. Mean filter indwell
time was 200.8 ± 156.9 days (median 160.0 days, range 5 – 736 days). The right internal jugular vein was used in
all retrieval procedures. The mean retrieval procedure time was 23.1 minutes and the mean fluoroscopy time was
6.3 minutes.
Venacavograms taken before and after the retrieval procedures of the IVC implant site revealed abnormalities
that the CEC determined to be related to the device in four patients. One patient had minimal, self limited contrast
extravasation post retrieval, one patient experienced intimal injury and caval narrowing of the IVC post retrieval,
one patient had minimal thrombus adjacent to the top of the filter prior to retrieval, and one patient had a failed
retrieval attempt due to clot burden with an abnormal appearance of the IVC . No clinical sequelae were reported
due to the retrieval procedure.
One hundred nineteen (119) of the 121 patients who had their filter retrieved completed one month follow-up and
two (2) patients were lost to follow-up. No instances of recurrent PE or new or worsening DVT were reported for
any patient completing the one month post-retrieval visit.
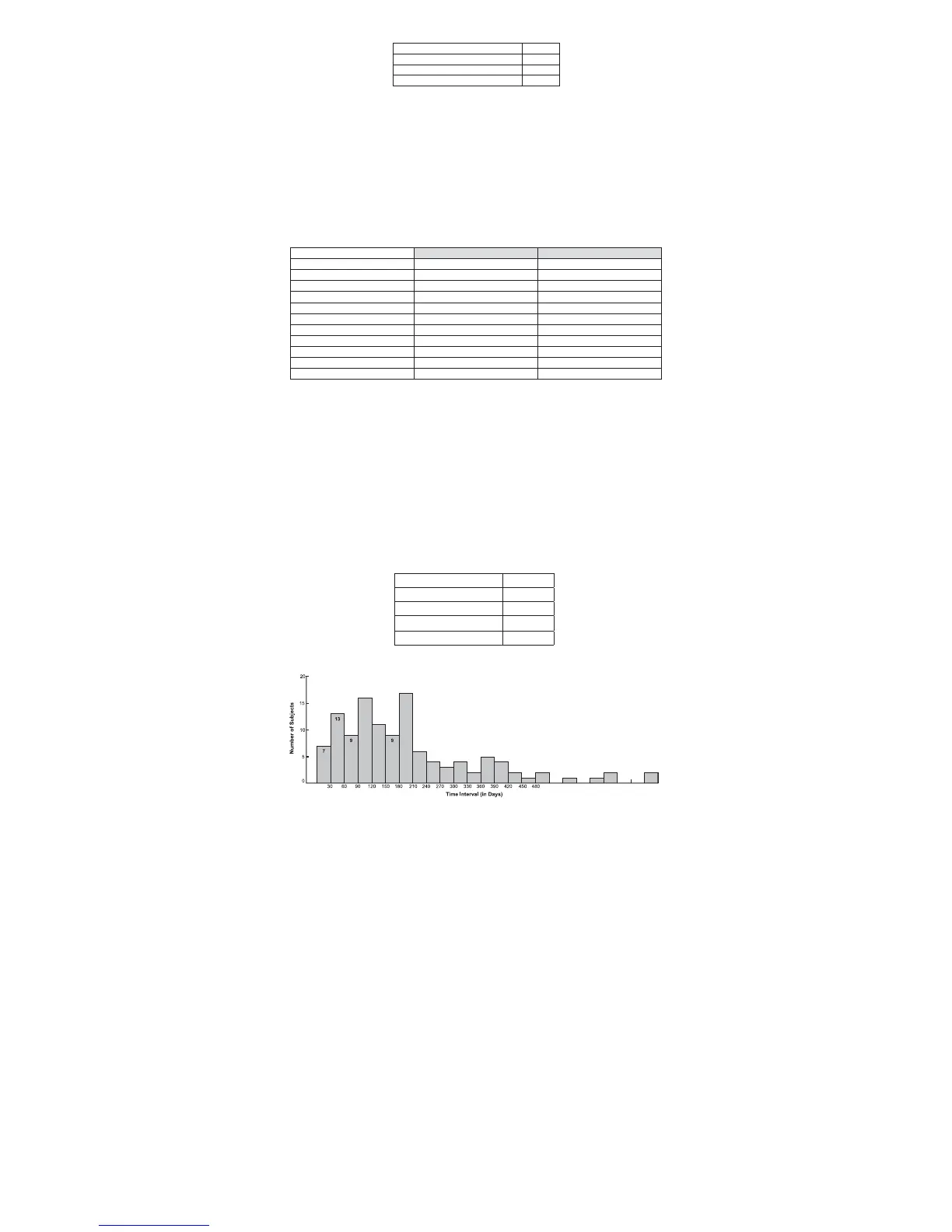
Table 4: D
ENALI
®
Filter Retrieval Details
Figure 3: Time from Implantation to Retrieval (N=121)
16
17
6
4
3
2
2
2
510 540 570 600 630 660 690 720
750
2 2
1001 100
44
5
11
I. Directions for Use - Implantation
1. Collect and prepare the following equipment for use.
• One D
ENALI
®
Vena Cava Jugular/Subclavian System that contains:
- One 55cm, 8.4 French I.D. introducer sheath and 8F dilator set
- One storage tube with preloaded D
ENALI
®
Filter and pusher
• 0.035" straight guidewire, 110cm long or longer
• 18 gauge entry needle
• Saline
• Contrast medium
• Syringe for saline infusion
• All basic materials for venipuncture: scalpel, #11 blade, local anesthesia, drapes, etc.
2. Select a suitable jugular or subclavian venous access route, on either the right or left side, depending upon the
patient’s size/anatomy, operator’s preference, or location of venous thrombosis. Right jugular/subclavian veins
are preferred.
3. Inspect the packaging to ensure that it has not been opened or damaged.
WARNING: Contents are supplied sterile. Do not use if the product sterilization barrier or its packaging is
compromised.
4. Prep, drape, and anesthetize the skin puncture site in standard fashion.
5. Open the inner pouch and remove the introducer sheath and both dilators using sterile technique.
6. Nick the skin with a #11 blade and perform venipuncture with an 18 gauge entry needle.
7. Insert the 0.035” straight guidewire and gently advance it into the inferior vena cava.
PRECAUTION: If resistance is encountered during the insertion procedure, withdraw the guidewire and
check vein patency fluoroscopically with a small injection of contrast medium. If a large thrombus is
present, remove the venipuncture needle and try the vein on the opposite side. A small thrombus may be
bypassed by the guidewire and introducer.
8. Remove the 18 gauge entry needle over the straight guidewire.
9. Flush the dilator and the introducer with saline. Insert the dilator through the introducer sheath ensuring that
the hubs connect properly. Advance the 8.4 French introducer sheath together with its tapered dilator over the
0.035" guidewire and into the inferior vena cava.
10. Remove the guidewire and perform a standard inferior venacavogram in both the AP and lateral view, (typically
30mL of contrast medium at 15mL/s) through the dilator. Check for caval thrombi, position of renal veins, and
congenital anomalies.
PRECAUTION: It is very important to maintain introducer patency with a saline flush to prevent occlusion
of the introducer which may interfere with the delivery device advancement.
WARNING: When injecting contrast medium through the dilator, do not exceed the maximum pressure
rating of 800 psi.
11. Select the optimum location (For example 1cm below the lowest renal) for filter placement and measure the IVC
diameter. IVC diameter may be measured using dilator radiopaque marker bands. Marker bands are spaced at a
distance of 28mm (outer-to-outer), which references the maximum indicated IVC diameter (Figure 2).
12. Reintroduce the guidewire and advance the introducer sheath with dilator to the selected optimum location
under flouroscopic guidance.
Number of Filter Retrieval Attempts 124
Number of Successful Retrievals 121
Retrieval Success Rate 97.6%
Mean Indwell Time 200.8 days
Maximum Indwell Time 736 days
 Loading...
Loading...