Do you have a question about the Dräger Isolette 8000 and is the answer not in the manual?
Ensure no patient is present, allow cooling, and use approved disinfection wipes for initial cleaning.
Follow specific instructions for disinfectant use, residue removal, drying, and component inspection.
Systematically remove components such as covers, reservoirs, and filters as part of the disassembly process.
Reassemble the device by installing components like filters, reservoirs, and covers in reverse order.
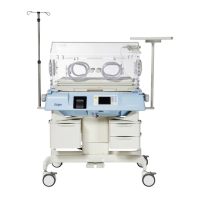
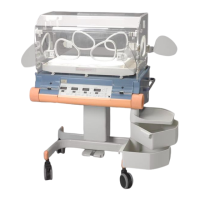
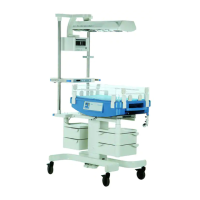
The Dräger Isolette 8000 / 8000 plus is an advanced infant incubator designed to provide a controlled and supportive environment for neonates. Its primary function is to maintain a stable temperature, humidity, and oxygen concentration, crucial for the healthy development and recovery of premature or critically ill infants. The device is engineered to offer a safe and nurturing space, mimicking the conditions of a mother's womb, thereby minimizing stress on the infant and facilitating optimal growth.
A key usage feature of the Isolette 8000 / 8000 plus is its intuitive interface, which allows caregivers to easily monitor and adjust environmental parameters. The system provides clear readouts of temperature, humidity, and oxygen levels, ensuring that the infant's needs are continuously met. The design incorporates features that enhance accessibility for medical staff, allowing for quick and efficient interventions without significantly disturbing the infant. This includes various access ports and a hood that can be raised, providing ample space for procedures while maintaining a controlled environment. The incubator also features an integrated scale, enabling precise weight monitoring without the need to remove the infant from the protective environment. An X-ray tray is another practical addition, allowing for imaging procedures to be performed directly within the incubator, further reducing the need for infant transfer and minimizing potential stress.
The Isolette 8000 / 8000 plus is built with a strong emphasis on patient safety and comfort. It includes mechanisms to prevent overheating and ensures a gentle airflow to avoid drafts. The materials used are selected for their biocompatibility and ease of cleaning, contributing to a hygienic environment for the infant. The device is also designed to be mobile, allowing for easy relocation within the hospital setting, which is essential for dynamic clinical environments.
Maintenance features of the Dräger Isolette 8000 / 8000 plus are designed to ensure thorough cleaning, disinfection, and operational reliability. The cleaning process is comprehensive, requiring the device to be switched off and allowed to cool down sufficiently before reprocessing begins. This ensures safety for the cleaning personnel and prevents damage to components. Disassembly is a key part of the maintenance routine, allowing access to all surfaces for effective cleaning and disinfection. Components such as gaskets, grommets, sleeves, sensor modules, the hood, mattress, scale, X-ray tray, bed, t-bars, upper cover, reservoir, and impeller cover are all designed for easy removal. This modular design facilitates a meticulous cleaning process, ensuring that all parts that come into contact with the infant or the internal environment can be properly sanitized.
The cleaning protocol emphasizes the use of approved disinfecting agents, applied to cleaning wipes rather than directly sprayed onto the device, especially near sensitive areas like the impeller fan. This prevents liquid penetration into the device, which could cause damage or malfunction. After disinfection, surfaces are wiped with a clean towel and water to remove any residue, followed by drying to prevent pooling or smear marks. This attention to detail in the cleaning process is critical for infection control in a neonatal care setting.
Regular inspection of all reprocessed components for damage or cracks is a crucial maintenance step before reassembly. This ensures that only intact and functional parts are put back into service, maintaining the device's integrity and safety. The reservoir, which is part of the humidification system, requires careful handling during disassembly and cleaning, as the water it contains can be hot. The air inlet filter is a replaceable component, indicating a routine maintenance task to ensure optimal air quality within the incubator. After reassembly, an operational checkout procedure is performed to confirm that the device operates correctly, ensuring it is safe and ready for therapy. The instructions also highlight the importance of using only disinfectants listed in the user manual and preventing liquid penetration into the device, reinforcing the commitment to both device longevity and patient safety.
| Category | Infant Incubator |
|---|---|
| Temperature Control | Yes |
| Humidity Control | Yes |
| Weight Capacity | 10 kg (22 lbs) |
| Oxygen Control Range | 21% |
| Air Filter | Yes |
| Noise Level | < 47 dBA |
| Power Supply | 100-240 V, 50/60 Hz |
| Height of Mattress (Adjustable) | Yes |
| Temperature Control Range (Skin Mode) | 34°C to 38°C (93°F to 100°F), can be extended to 39°C (102°F) |
| Humidity Control Range | 30% to 95% relative humidity (RH) |





 Loading...
Loading...