Information
1-1. DVT-2600 Introduction
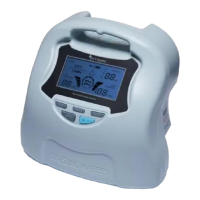
Thank you for choosing the DVT-2600. This product is a specialized Intermittent
pneumatic compression (IPC) system designed to prevent DVT (Deep Vein Thrombosis)
and PE (Pulmonary Embolism) by improving venous blood flow to a patient who has a
risk factor of DVT/PE.
This system consists of the device, tubing sets and sleeves. The DVT-2600 offers
sequential inflation which promotes venous blood movement and metabolism. After
deflation, there is a time interval before reinflation to allow the veins to refill. The
operation of the inflation and deflation is repeated until the stop button is activated.
The DVT-2600 has an integrated self-checking system which includes checks for the
digital sensor, sleeve connection, and power supply. If an error code shows during initial
operation of the DVT-2600, it can be checked against the error code key attached to the
side of the DVT-2600 unit for convenient reference.
1-2. Intended use
A device intended to prevent DVT/PE by increasing venous blood flow to a patient who
has a risk factor of DVT/PE.
1-3. Target treatment group of disease
- Pulmonary embolism
- Deep vein thrombosis
1-4. Expected except group of treatment
Do not use this product if you have any of the following conditions:
- Pre-existing deep vein thrombosis, phlebothrombosis or pulmonary embolism
- Presumptive evidence of Congestive Heart Failure
- Inflammatory Phlebitis Process
- Severe arteriosclerosis or other ischemic vascular disease
- Decompensated cardiac insufficiency
- Carcinoma metastasis in the affected Extremity
- Lymphatic return is undesirable
- Severe arteriosclerosis or active infection
1
8
 Loading...
Loading...