i-16 Ultrasound System – Common Service Information
Direction 5444964-100 English
Rev. 5
Omission and errors
If there are any omissions, errors or suggestions for improving
this documentation, contact the GE Ultrasound Global
Documentation Group with specific information listing the
system type, manual title, part number or direction number,
revision number, page number and suggestion details.
Mail the
information to:
GE
Service Documentation
9900 Innovation Drive (RP-2156)
WAUWATOSA, WI 53226
USA
GE employees should use Post-Market Quality Management
(PQM) to report service documentation issues.
Service Safety Considerations
For a complete review of all safety requirements, see: ‘Safety
Information’ on page 2-1.
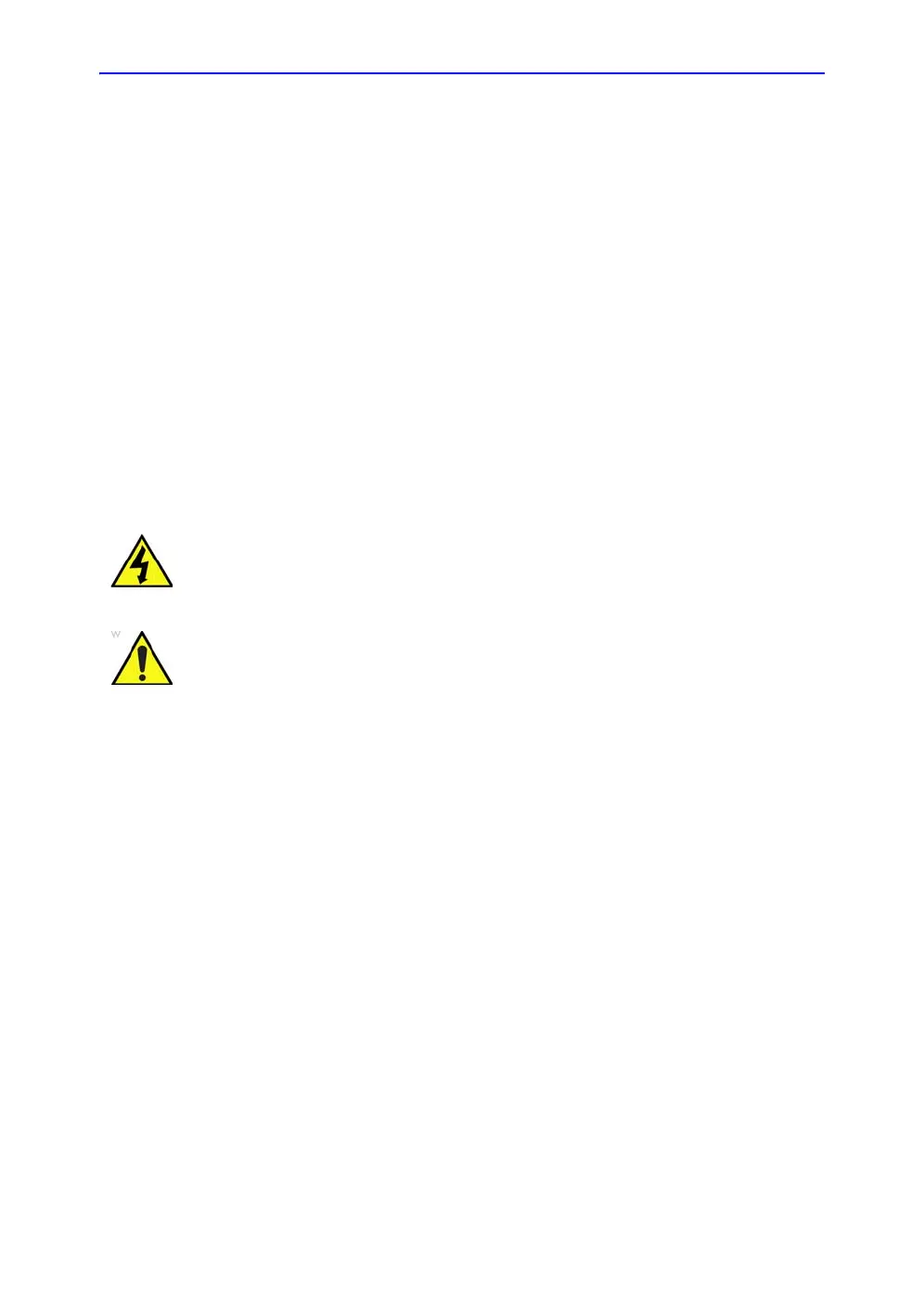
DANGER DANGEROUS VOLTAGES, CAPABLE OF CAUSING DEATH, ARE PRESENT
IN THIS EQUIPMENT. USE EXTREME CAUTION WHEN HANDLING,
TESTING AND ADJUSTING.
WARNING
Use all Personal Protection Equipment (PPE) such as gloves,
safety shoes, safety glasses, and kneeling pads, to reduce the
risk of injury.
 Loading...
Loading...