Care and Maintenance
4-34 Ultrasound System – Common Service Information
Direction 5444964-100 English
Rev. 5
Isolated lead (sink) leakage test record (continued)
Keep a record of the results with other hard copies of
maintenance data using Table 4-14.
NOTE: Not all test procedures are applicable to all areas of the world.
Reversed Polarity testing content satisfies regions following IEC
62353:2007 and IEC 60601-1:2005.
NOTE: Values in italics font are given as examples only.
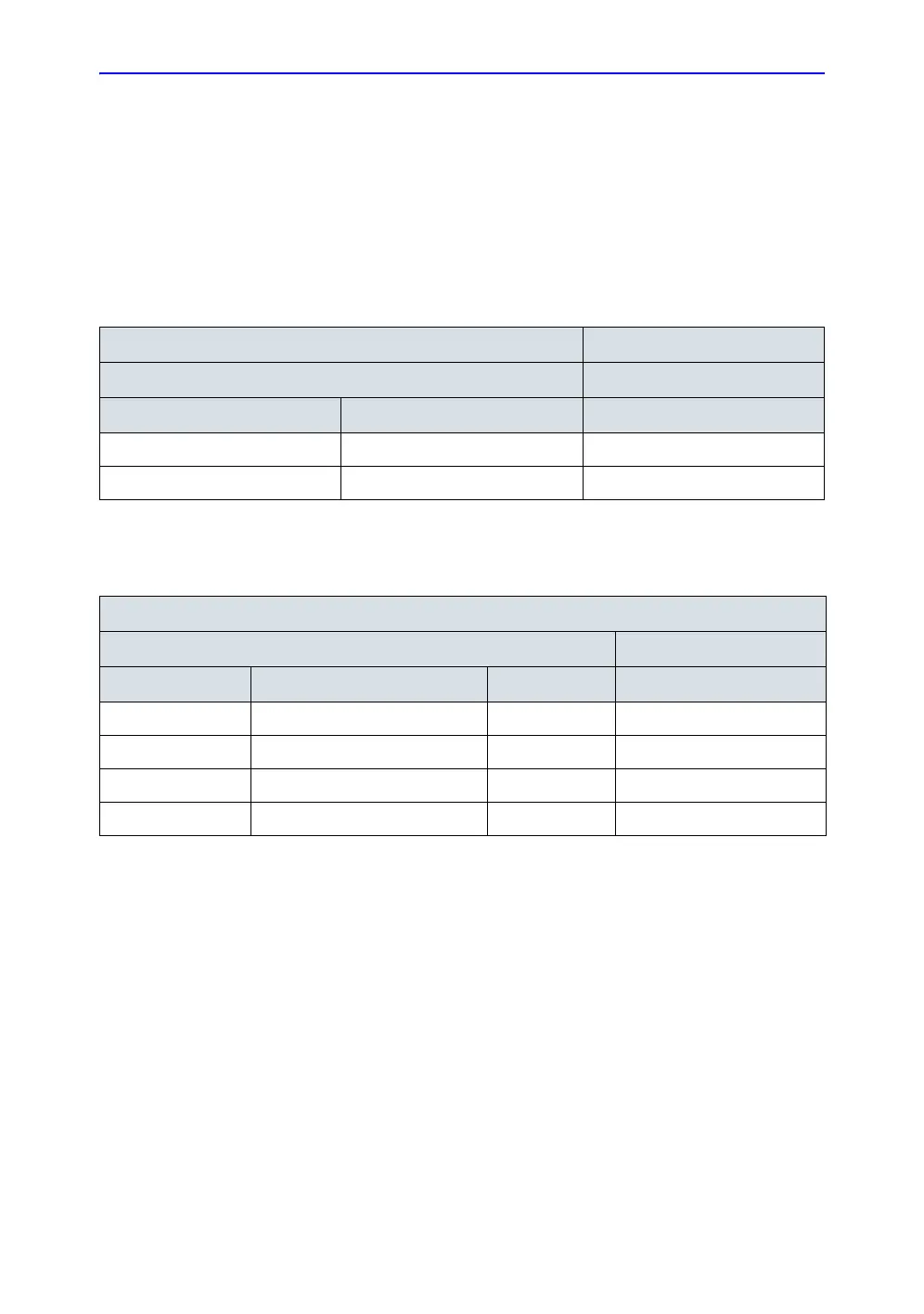
Table 4-14: Typical data format for recording isolated patient lead (sink) leakage
Unit under test_________________________________ Date of test:___________
Test Conditions Patient Lead
System Power Grounding/PE RA+LA+LL
on closed
off closed
Table 4-15: Typical data format for recording isolated patient lead (sink) leakage
Unit under test____________________________________ Date of test:_____________
Test Conditions Patient Lead
System Power Grounding/PE Limit µA RA+LA+LL
off closed 50
on closed 50
off closed (reversed polarity) 50
on closed (reversed polarity) 50
 Loading...
Loading...