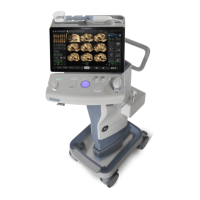
REGULATORY REQUIREMENTS
This product complies with the regulatory requirements of the following:
D Council Directive 93/42/EEC concerning medical devices: the
0459
label affixed
to the product testifies compliance to the Directive.
For a system, the location of the CE marking label is described in the system manual.
European registered place of business:
GE Medical Systems Europe
Quality Assurance Manager
BP 34
F 78533 BUC CEDEX France
Tel: +33 (0)1 30 70 40 40
D Medical Device Good Manufacturing Practice Manual issued by the FDA (Food and
Drug Administration, Department of Health, USA).
D Underwriters’ Laboratories, Inc. (UL), an independent testing laboratory.
D Canadian Standards Association (CSA).
D International Electrotechnical Commission (IEC), international standards organiza-
tion, when applicable.
D USA/HHS:
United States Federal law restricts this device to use by or on the order of a
physician.
D General Electric Medical Systems is ISO 9001 and EN 46001 certified.
D The original document was written in English.
FOR TRAINING PURPOSES ONLY!
NOTE: Once downloaded, this document is UNCONTROLLED, and therefore may not be the latest revision. Always confirm revision status against a validated source (ie CDL).
 Loading...
Loading...