6
6
ENGLISH
recommended Software upgrades; or (d) designated as supplied
subject to a non-Hologic warranty or on a pre-release or “as-is”
basis.
TECHNICAL SUPPORT AND PRODUCT RETURN INFORMATION
Contact Hologic Technical Support if the Omni Hysteroscope
System fails to operate as intended. If product is to be returned
to Hologic for any reason, Technical Support will issue a Returned
Materials Authorization (RMA) number and biohazard kit if
applicable. Return the Omni Hysteroscope System according to the
instructions provided by Technical Support. Be sure to clean and
sterilize the product before returning it and include all accessories
in the box with the returned unit.
Return used or opened product according to the instructions
provided with the Hologic-supplied biohazard kit.
For More Information
For technical support or reorder information in the United States,
please contact:
Hologic, Inc.
250 Campus Drive
Marlborough, MA 01752 USA
Phone: 1.800.442.9892 (toll-free)
www.hologic.com
International customers, contact your distributor or local Hologic
Sales Representative:
European Representative
Hologic Ltd.
Heron House Oaks Business Park, Crewe Road
Wythenshawe, Manchester. M23 9HZ, UK
Phone: +44 (0)161 946 2206
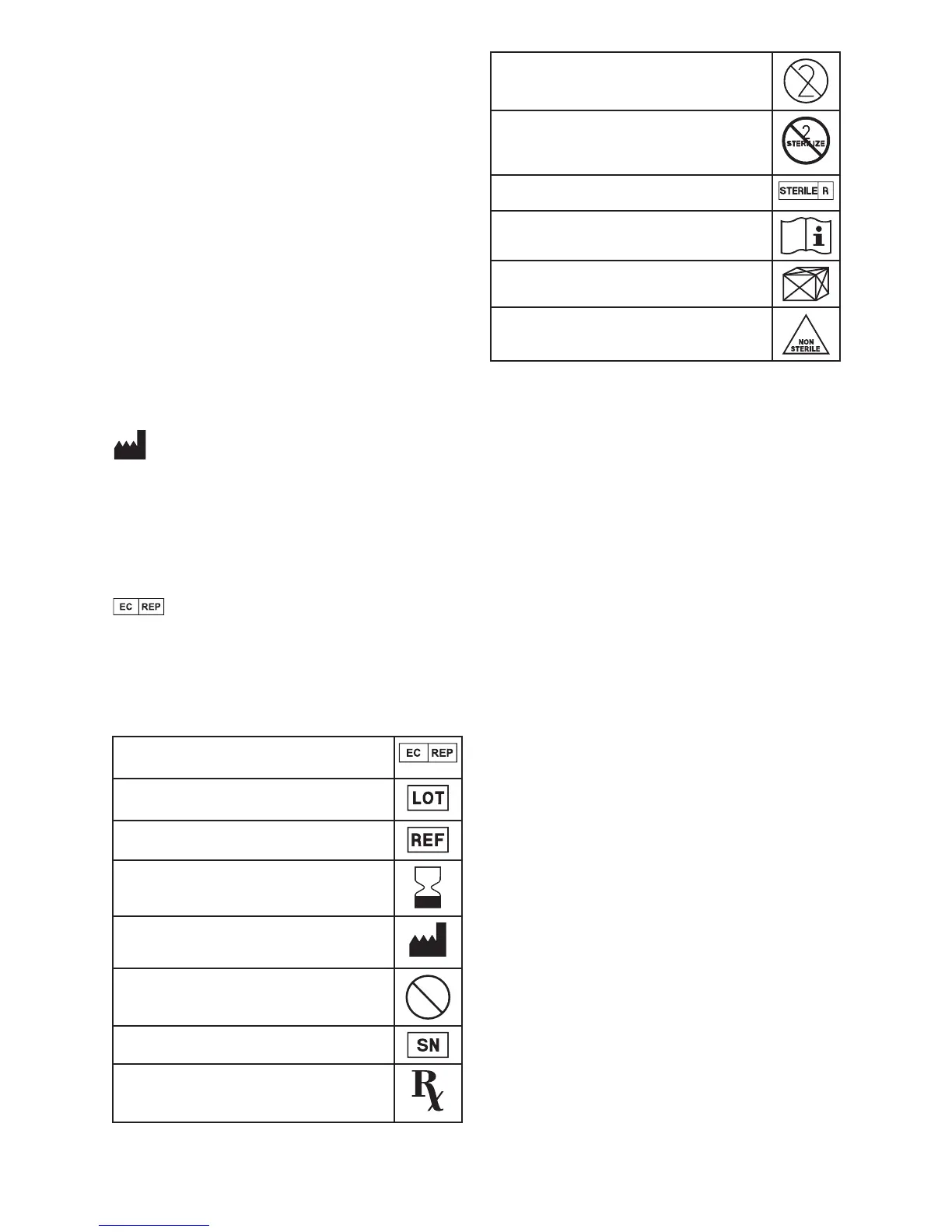
Symbols Used on Labeling
Authorized Representative in the European Community
Batch code
Catalogue number
Use by
Manufacturer
Patient contact parts do not contain phthalate
DEHP
Serial number
U.S. federal law restricts this device to sale by or on the order of
a physician
Do not re-use
Do not resterilize
Sterilized using irradiation
Consult instructions for use
Contents
Non-sterile
Hologic, Omni, and MyoSure are associated logos are registered
trademarks of Hologic, Inc. and/or its subsidiaries in the United
States and other countries. All other trademarks, registered
trademarks, and product names are the property of their respective
owners.
© 2018 Hologic, Inc.
AW-17817-002 Rev. 006
 Loading...
Loading...