18
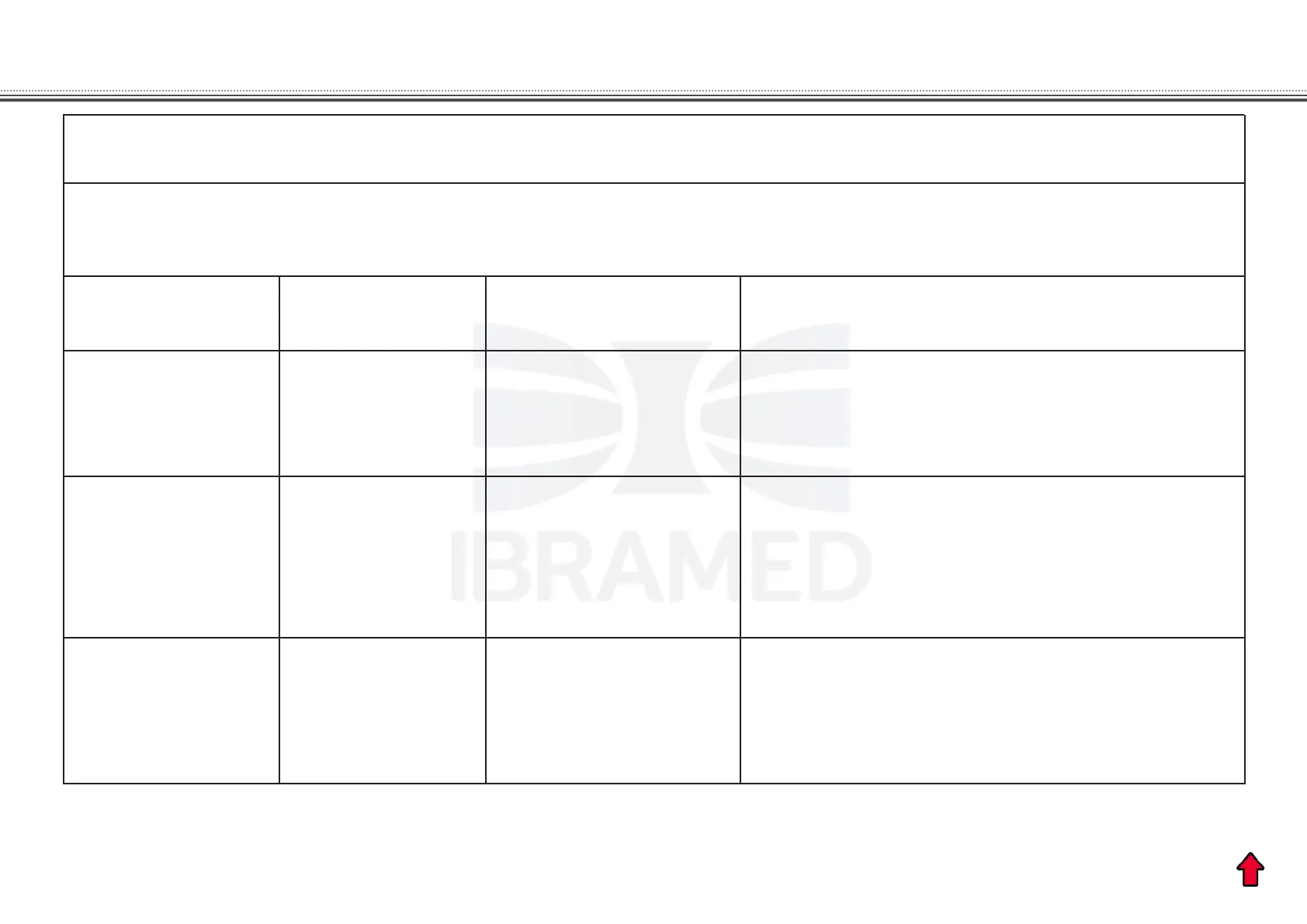
Manufacturer’s guidelines and declaration – Electromagnetic immunity
Immunity Test Conformity level
Electromagnetic environment – orientations
Test level
IEC 60601
Electrostatic
discharge (ESD)
IEC 61000-4-2
± 6 kV by contact
± 8 kV by air
± 6 kV by contact
± 8 kV by air
± 2 kV in the
feeding lines
± 1 kV in the
input/output lines
± 2 kV in the
feeding lines
± 1 kV in the
input/output lines
± 1 kV dierential
mode
± 2 kV common
mode
± 1 kV dierential
mode
± 2 kV common
mode
Fast electric
transitories /
pulse train
(Burst)
IEC 61000-4-4
The quality of power supply should be that of
a hospital environment a or typical commercial
building.
The quality of power supply should be that of a
typical commercial or hospital environment.
Outbreaks
IEC 61000-4-5
The oor should be wooden, concrete or ceramic.
If oors are covered with synthetic material,
the relative humidity should be at least 30%.
SONOPULSE III is destined to be used in the electromagnetic environment specied below. The user of the equipment
should ensure that it is used in such environment.
ELECTROMAGNETIC COMPATIBILITY GUIDANCE
 Loading...
Loading...