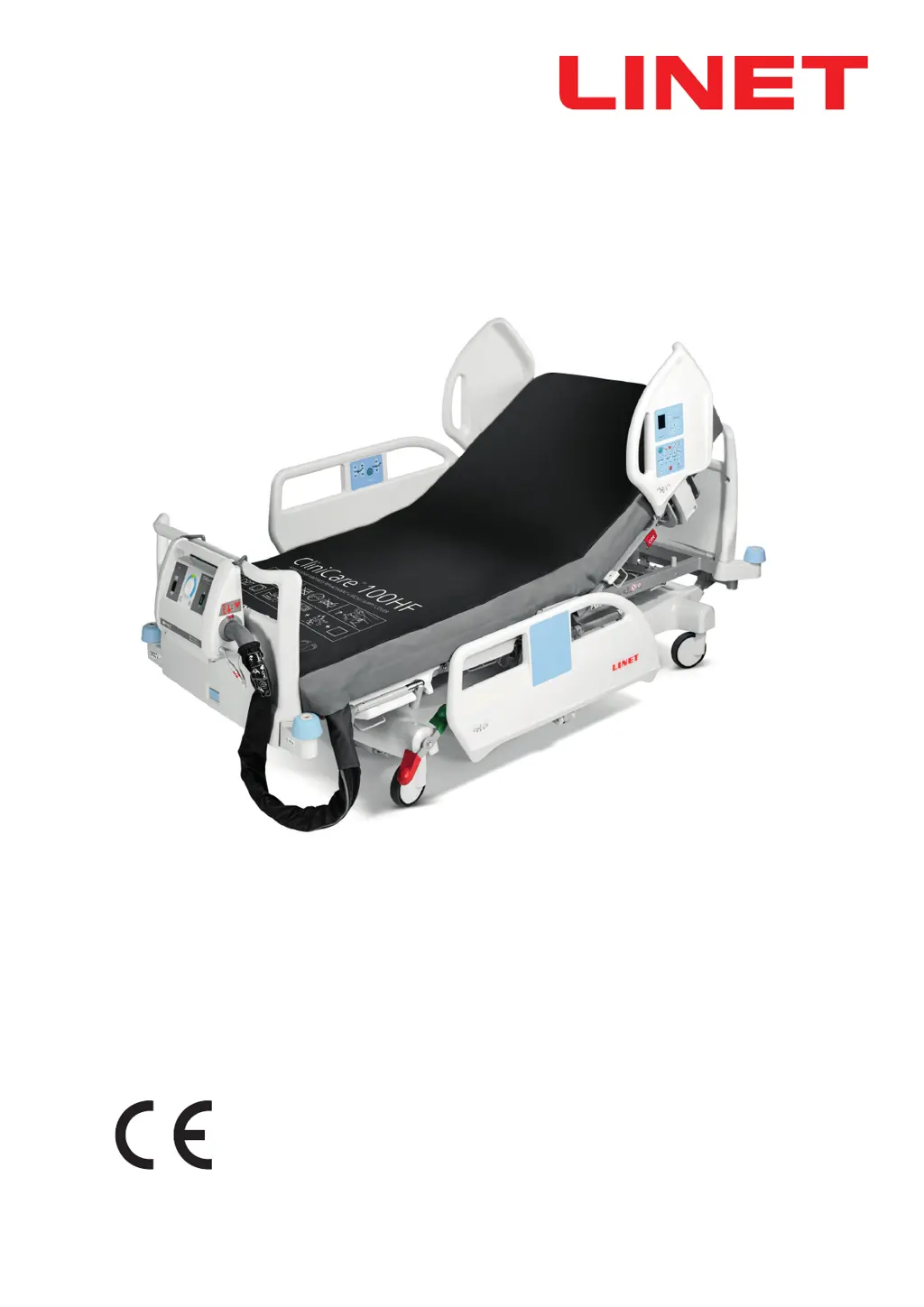
How to troubleshoot power failure on LINET Medical Equipment?
- KKatrina FieldsAug 6, 2025
If your LINET Medical Equipment SCU will not turn on, first ensure the Mains switch on the SCU is in the on ( I ) position. Then, check that the SCU is connected to a functioning electrical wall socket.