7 Sterilization
32
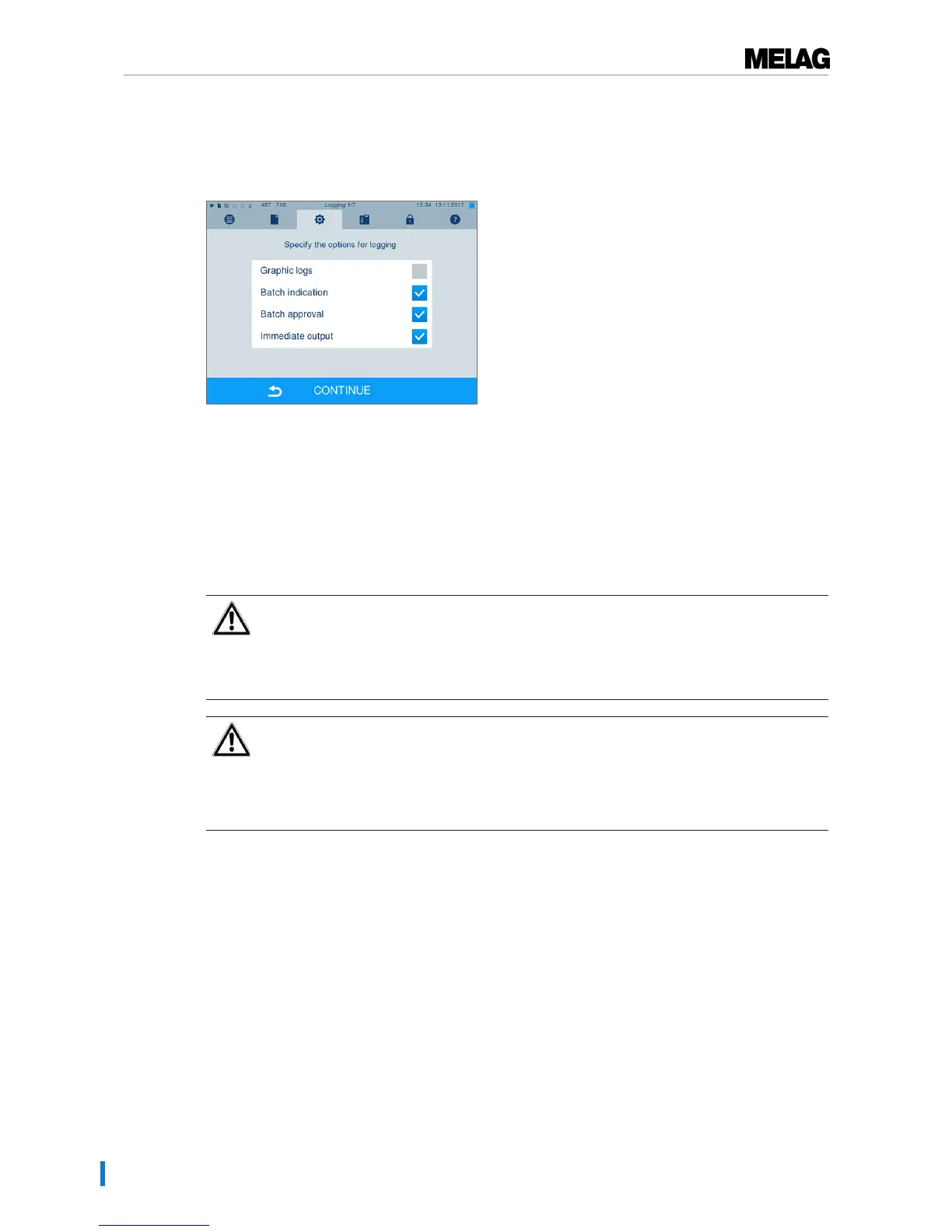
The approval process
According to RKI "Hygiene requirements for the treatment of medical products", instrument treatment ends
with the documented approval for storage and application of the sterilized equipment. The approval
process consists of batch indication and batch approval and must be performed by authorized and expert
personnel.
Batch indication comprises the checking of the indicators used in the sterilization program e.g.
MELAcontrol/MELAcontrol PRO. Approval of the indicator strip is possible only if it changes colour entirely.
Batch approval comprises the checking of the process parameters using the sterilization results on the
steam sterilizer and the sterilization log as well as checking of the individual packaging for damage and
residual moisture. The sterilization log records the approval of the batch and any indicators. Depending on
the setting in the user administration, approval for the sterilized equipment requires the user PIN of the
person who provides approval for the batch and the indicators.
Removing the sterilized equipment
CAUTION
Danger of burns from hot metal surfaces
n Allow the device to cool sufficiently before opening.
n Do not touch any hot metal parts.
CAUTION
Unsterile instruments resulting from damaged or burst packaging. This endangers the
health of your patients and practice team.
n Should the packaging be damaged or have burst, re-pack the sterilization material and re-
sterilize it.
If you remove the sterilized equipment from the device directly after the end of the program, it is possible
that the instruments can be partially damp. According to the Arbeitskreis für Instrumentenaufbereitung
(AKI; red broschure 10. Edition; S.57): "In practice, residual moisture in the form of a few drops of water
capable of evaporating within 15 minutes is tolerated, but actual pools of water are not acceptable."
Comply with the following specifications when removing the sterilized equipment:
u Never use force to open the door. This could damage the device and / or result in the emission of hot
steam.
u Hold the mount level when removing it from the steam sterilizer. Otherwise, the load could slide off.
u When removing the load from the steam sterilizer separately, ensure that the mount does not slide out
unintended.
u Use a tray lifter to remove the tray.
u Never touch the sterilized equipment, the device interior or the inside of the the door with unprotected
hands. The components are hot.
u Check the packaging on the sterilized equipment for damage when removing it from the device.
Should the packaging be damaged, re-pack the sterilization material and re-sterilize it.

 Loading...
Loading...











