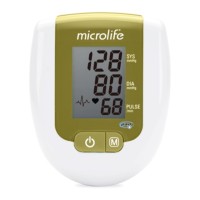
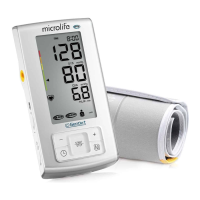
8
10.Technical specifications
This device complies with the requirements of the Medical Device
Directive 93/42/EEC.
Technical alterations reserved.
Operating condi-
tions:
10 - 40 °C / 50 - 104 °F
15-90 % relative maximum humidity
Storage conditions: -20 - +55 °C / -4 - +131 °F
15-90 % relative maximum humidity
Weight: 277 g (including batteries)
Dimensions: 131 x 90 x 60.5 mm
Cuff size: from 17 - 52 cm according to the cuff sizes
(see «Selecting the correct cuff»)
Measuring proce-
dure:
oscillometric, corresponding to Korotkoff
method: Phase I systolic, Phase V
diastolic
Measurement range: 20 - 280 mmHg – blood pressure
40-199 beats per minute – pulse
Cuff pressure display
range: 0 - 299 mmHg
Resolution: 1 mmHg
Static accuracy: within ± 3 mmHg
Pulse accuracy: ± 5 % of the readout value
Voltage source:
4 x 1.5 V alkaline batteries; size AA
Mains adapter DC 6V, 600 mA (optional)
Battery lifetime: approx. 920 measurements
(using new batteries)
IP Class: IP 20
Reference to
standards:
EN 1060-1 /-3 /-4; IEC 60601-1;
IEC 60601-1-2 (EMC); IEC 60601-1-11
Expected service life: Device: 5 years or 10000 measurements,
whichever comes first
Accessories: 2 years or 5000 measure-
ments, whichever comes first
 Loading...
Loading...